| Human Metabolome Technologies (6090) |
|
||||||||
Company |
Human Metabolome Technologies Inc. |
||
Code No. |
6090 |
||
Exchange |
Mothers of Tokyo Stock Exchange |
||
Industry |
Service Industry |
||
President |
Ryuji Kanno |
||
Address |
246-2 Mizukami, Kakuganji, Tsuruoka-city, Yamagata |
||
Year-end |
End of March |
||
URL |
|||
*Share price as of closing on the end of June 20. Number of shares outstanding, ROE and BPS are based on actual results of the previous term end.
|
||||||||||||||||||||||||
|
|
* Forecasts are those of the Company. From the current fiscal year, the definition for net income has been changed to net income attributable to parent company shareholders (Abbreviated as parent net income).
|
| Key Points |
 |
| Company Overview |
|
Human Metabolome Technologies Inc. is a venture company originated from Keio University, which main focuses are: a) commissioned metabolome analysis service for research institutions and pharmaceutical companies and b) biomarker development. Its metabolome analysis technology is highly recognized throughout the world as a basic methodology to identify biomarkers. Its business model is to steadily generate profit through the commissioned metabolome analysis business while investing in and working on research and development of biomarkers which estimates future prospects. The company is pursuing continuous growth with a stable revenue base under this strategy.
On the other hand, close attention must be paid to the steady expansion of the metabolome analysis business.
Although the company completed its fourth fiscal year since getting listed on December 2013, the total operating CF for the four periods was a surplus 82 million yen. Although it is not a substantial amount, compared to other biotechnology venture, which has nearly a billion yen deficit every term, we can see the advantage of the company with a metabolome analysis business that is steadily profitable.
 In 2001, Professor Tomoyoshi Soga of Keio University, Institute for Advanced Biosciences, developed a system to measure broad species of small-molecule metabolites (metabolome), which is called the CE-MS system. The new innovative system realized to capture a wide range of metabolites at once, while none of the conventional approaches do not, which means it used to be necessary to involve multiple experimental conditions, each of which has a small coverage.
Metabolome analysis techniques had been used for various purposes such as fundamental biological research, development of pharmaceutical products, disease biomarkers, etc., and were expected to expand the demand in the future. Therefore, upon establishment of the CE-MS system, Human Metabolome Technologies Inc. was established in July 2003 to commercialize the method chiefly by Professor Soga, Professor Masaru Tomita, also from Keio University, and the office of Keio University. It was the first venture company originated from Keio University, with a financial support from Keio University's entrepreneurship fund.
In 2008, Mr. Ryuji Kanno became the president of the Company. Before taking up this position, Mr. Kanno who was the Vice President & CEO of Agilent Technologies Japan, Ltd., a Japanese subsidiary of Agilent Technologies, a global company that develops, manufactures, sells, and support chemical analysis equipment and electric & electronic measurement equipment in the field of life science, which, had been for some time in business relationships with HMT and Keio University.
President Kanno promoted research and development of the Company's core technologies and also began organizing and establishing more specific commercialization process and business models. At the same time, he began preparation for the Company to be listed on the stock market in order to accelerate its growth speed through enhancing the Company's visibility and raising funds for research and development. The Company got listed on the Mothers Section of the Tokyo Stock Exchange in December 2013, ten years after its establishment.
【Corporate Philosophy】
The Company defines the meaning of its existence as follows.
"To contribute to people's health and joyful lives through research and development using the up-to-date metabolome analysis technologies for children in the future."
The Company also sets the following 5 "Common Values
In 2001, Professor Tomoyoshi Soga of Keio University, Institute for Advanced Biosciences, developed a system to measure broad species of small-molecule metabolites (metabolome), which is called the CE-MS system. The new innovative system realized to capture a wide range of metabolites at once, while none of the conventional approaches do not, which means it used to be necessary to involve multiple experimental conditions, each of which has a small coverage.
Metabolome analysis techniques had been used for various purposes such as fundamental biological research, development of pharmaceutical products, disease biomarkers, etc., and were expected to expand the demand in the future. Therefore, upon establishment of the CE-MS system, Human Metabolome Technologies Inc. was established in July 2003 to commercialize the method chiefly by Professor Soga, Professor Masaru Tomita, also from Keio University, and the office of Keio University. It was the first venture company originated from Keio University, with a financial support from Keio University's entrepreneurship fund.
In 2008, Mr. Ryuji Kanno became the president of the Company. Before taking up this position, Mr. Kanno who was the Vice President & CEO of Agilent Technologies Japan, Ltd., a Japanese subsidiary of Agilent Technologies, a global company that develops, manufactures, sells, and support chemical analysis equipment and electric & electronic measurement equipment in the field of life science, which, had been for some time in business relationships with HMT and Keio University.
President Kanno promoted research and development of the Company's core technologies and also began organizing and establishing more specific commercialization process and business models. At the same time, he began preparation for the Company to be listed on the stock market in order to accelerate its growth speed through enhancing the Company's visibility and raising funds for research and development. The Company got listed on the Mothers Section of the Tokyo Stock Exchange in December 2013, ten years after its establishment.
【Corporate Philosophy】
The Company defines the meaning of its existence as follows.
"To contribute to people's health and joyful lives through research and development using the up-to-date metabolome analysis technologies for children in the future."
The Company also sets the following 5 "Common Values
 【Points to Understand the Company】
The Company's business overview is described later in this report together with the explanation of key words, "metabolomics" and "biomarker", however, many technical terms show up in the section, and that may prevent the readers from understanding. Here are three points briefly provided before jumping to the section to glancing at the Company's business scope.
① Significance of Social Presence
Biomarkers are in vivo substances that are used as indicators to assess the current state of specific diseases. Blood sugar" for diabetes, "γ-GPT" for liver function disorder, and "uric acid" for gout are the representative examples of well-known and widely used biomarkers.
The Company discovered a biomarker for "major depressive disorder", one of the current major social issues, and is developing diagnostic agents to quantify the condition of the disease.
Because there is no prevailing method to objectively measure the status of depression, despite the increase in the number of patients with depression, some serious problems are emerging regarding the therapy of depression. For example, patients who would have been cured if they had properly treated are still suffering or in overprescription. If the diagnostic agents using the Company's biomarker become to be widely used, these issues are expected to be solved, and social loss will be subsequently reduced. This social significance cannot be overlooked to understand the Company.
② Excellent Technological Capacities
The Company is highly recognized thanks to the "metabolome analysis technology" which is used to examine the complicated behavior of metabolites in human bodies to identify biomarkers.
The biomarker for depression is just an example. In Oct. 2017, the biomarker for acute encephalopathy was patented in Japan. With the technology, the Company is expected to identify and develop various new biomarkers in the future.
③ Stable Business Model
The Company's current core business is the "commissioned metabolome analysis business", supporting research and development activities of research institutions and pharmaceutical companies that occupy the greater part of its sales. Sales of FY March 2018 were 936 million and operating income was 445 million, showing steady income.
On the other hand, the "biomarker business", which is expected to achieve significant growth in the mid- to long-term, is still operated in a small scale and experiencing losses. However, the Company has already established a balanced business model, in which the profit generated from the commissioned metabolome analysis business is invested into the biomarker business for its growth. This model is notable among many bio-venture companies that are suffering from gaining profit.
【Depression】
In the "biomarker business", the Company's future growth driving force, the Company is currently targeting depression. Below is the overview of depression and major depressive disorder and their situation in Japan.
◎What is Depression?
Depression is a type of mood disorder and is a brain dysfunctional state for various reasons such as accumulated physical and mental stress. Because the brain is not functioning properly, people in the depressed mood feel negative and low-esteem. This causes a vicious cycle in which people with depression feel more stress for matters that they could otherwise handle.
The "major depressive disorder" refers to the state in which depression mood continues even after the sources of stress are removed. In this regard, it is distinguished from adjustment disorders or some anxiety disorders and is considered a brain dysfunction, instead of a mere response to stress.
(Note: "Major" of the major depressive disorder means "primary" and does not mean "serious" depression.)
◎Number of depression patients in World and Japan
In 2012, the World Health Organization (WHO) announced that at least 350 million people were with depression which is a mental disorder. Almost 1 million lives are lost due to suicide every year, and over half of them are assumed to be due to depression.
According to the "Patient Survey" conducted by the Ministry of Health, Labor, and Welfare (MHLW) every three years targeting health facilities across the country, the total number of patients with mood disorders including depression increased from 430,000 in 1996 to 950,000 in 2011 (2.2x). The "Patient Survey" shows statistical data of the number of patients who visit health facilities. It is known that the consultation rate of patients with depression is low. Thus, according to the MHLW, it is suspected that the actual number of depression patients might be larger. 【Points to Understand the Company】
The Company's business overview is described later in this report together with the explanation of key words, "metabolomics" and "biomarker", however, many technical terms show up in the section, and that may prevent the readers from understanding. Here are three points briefly provided before jumping to the section to glancing at the Company's business scope.
① Significance of Social Presence
Biomarkers are in vivo substances that are used as indicators to assess the current state of specific diseases. Blood sugar" for diabetes, "γ-GPT" for liver function disorder, and "uric acid" for gout are the representative examples of well-known and widely used biomarkers.
The Company discovered a biomarker for "major depressive disorder", one of the current major social issues, and is developing diagnostic agents to quantify the condition of the disease.
Because there is no prevailing method to objectively measure the status of depression, despite the increase in the number of patients with depression, some serious problems are emerging regarding the therapy of depression. For example, patients who would have been cured if they had properly treated are still suffering or in overprescription. If the diagnostic agents using the Company's biomarker become to be widely used, these issues are expected to be solved, and social loss will be subsequently reduced. This social significance cannot be overlooked to understand the Company.
② Excellent Technological Capacities
The Company is highly recognized thanks to the "metabolome analysis technology" which is used to examine the complicated behavior of metabolites in human bodies to identify biomarkers.
The biomarker for depression is just an example. In Oct. 2017, the biomarker for acute encephalopathy was patented in Japan. With the technology, the Company is expected to identify and develop various new biomarkers in the future.
③ Stable Business Model
The Company's current core business is the "commissioned metabolome analysis business", supporting research and development activities of research institutions and pharmaceutical companies that occupy the greater part of its sales. Sales of FY March 2018 were 936 million and operating income was 445 million, showing steady income.
On the other hand, the "biomarker business", which is expected to achieve significant growth in the mid- to long-term, is still operated in a small scale and experiencing losses. However, the Company has already established a balanced business model, in which the profit generated from the commissioned metabolome analysis business is invested into the biomarker business for its growth. This model is notable among many bio-venture companies that are suffering from gaining profit.
【Depression】
In the "biomarker business", the Company's future growth driving force, the Company is currently targeting depression. Below is the overview of depression and major depressive disorder and their situation in Japan.
◎What is Depression?
Depression is a type of mood disorder and is a brain dysfunctional state for various reasons such as accumulated physical and mental stress. Because the brain is not functioning properly, people in the depressed mood feel negative and low-esteem. This causes a vicious cycle in which people with depression feel more stress for matters that they could otherwise handle.
The "major depressive disorder" refers to the state in which depression mood continues even after the sources of stress are removed. In this regard, it is distinguished from adjustment disorders or some anxiety disorders and is considered a brain dysfunction, instead of a mere response to stress.
(Note: "Major" of the major depressive disorder means "primary" and does not mean "serious" depression.)
◎Number of depression patients in World and Japan
In 2012, the World Health Organization (WHO) announced that at least 350 million people were with depression which is a mental disorder. Almost 1 million lives are lost due to suicide every year, and over half of them are assumed to be due to depression.
According to the "Patient Survey" conducted by the Ministry of Health, Labor, and Welfare (MHLW) every three years targeting health facilities across the country, the total number of patients with mood disorders including depression increased from 430,000 in 1996 to 950,000 in 2011 (2.2x). The "Patient Survey" shows statistical data of the number of patients who visit health facilities. It is known that the consultation rate of patients with depression is low. Thus, according to the MHLW, it is suspected that the actual number of depression patients might be larger.
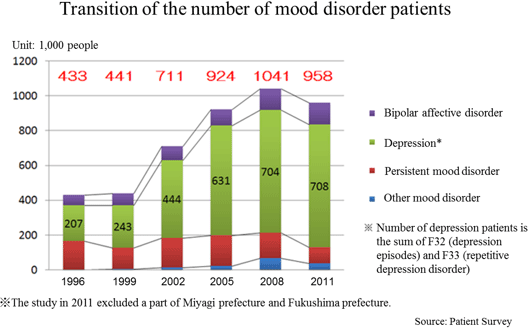 It is said that majority of depression patients are women and young people, however, in Japan, it is also frequently found among middle-aged people, and the social and economic impact of depression is significant. Needless to say that depression distresses both patients and their families, and its social aspects cannot be passed over either; it leads reduction on the productivity of organizations and even suicide.
In Japan, economic losses due to depression and suicide are estimated to be about 3 trillion yen. If there were no such losses, GDP would increase about 2 trillion yen (an estimate by the Ministry of Health, Labour and Welfare, 2010).
Economic losses around the world are estimated to be about 62 trillion yen in 2002 (Screening for Depression in Adults: A Summary of the Evidence. Ann Intern Med. 2002.). The current economic losses are estimated to be over 100 trillion yen.
◎Treatment of Depression
If someone is diagnosed as having depression, the common treatment is prescription of "antidepressant drugs." The antidepressant drugs can be categorized into several groups such as selective serotonin reuptake inhibitor (SSRI), serotonin-noradrenaline reuptake inhibitor (SNRI), and tricyclic antidepressant. In addition, anti-anxiety drugs or sleep inducing drugs can be used, depending on the symptom.
The important thing under drug therapy is that the patients comply with the prescribed amount and frequency after they are informed about the effects and side effects. However, patients with depression often reduce the amount or frequency without doctors' permission as they do not feel the symptom to be very serious, they worry side effects, etc. In these cases, the patients do not show improvement as doctors expect, and the doctors prescribe more drugs or change the types of drugs. That would often result in the delay of recovery or excessive administration of drugs, for lack of trust between doctors and patients. Therefore, it is essential to have objective assessment standards quantifying the state of depression or proving the depression has been cured. The biomarker and diagnostic agents of the depression that the Company is currently working on are extremely important for prompt and appropriate treatment of the disease.
【Metabolomics and Biomarker】
In order to understand the outline of the Company's business activities, it is necessary to have a certain understanding of two key words, "metabolomics" and "biomarker".
<What is metabolomics?>
Living organisms including human beings consist of some parts with various functions such as muscles, internal organs, and bones. "Metabolites" such as amino acids, fatty acids, and nucleic acids are common and major components of these organs. Metabolites play crucial roles for entire life activities.
Metabolites are provided by food and are consumed in the process of daily actions such as exercise. They move in a body and cells in accordance with their functions and are converted into new substances through various chemical reactions, which are called "metabolism". Adjusting body temperature, breathing, moving heart, digesting and absorbing food, transforming old cells into new ones are all operated by metabolism. The "substance conversion" to a new substance is based on a certain flow called metabolic pathway.
One of well-known approaches to understand the mechanisms of a human body is "genomics", analyzing genes. Now, automated sequencing and computer analysis of genetic information (DNA base sequences) is available, and nearly all the information in the human genome has already been deciphered. However, much about the relationship between the roles of genes and diseases remains unknown. Recently, more researchers lean towards investigating metabolic profiles, in addition to genetic information coming out of genome analysis, in order to understand the relationships between a human body and diseases. Consequently, research and use of "metabolomics (metabolome analysis)" targeting all metabolites is becoming increasingly popular.
The metabolome analysis is mainly used in the following fields:
It is said that majority of depression patients are women and young people, however, in Japan, it is also frequently found among middle-aged people, and the social and economic impact of depression is significant. Needless to say that depression distresses both patients and their families, and its social aspects cannot be passed over either; it leads reduction on the productivity of organizations and even suicide.
In Japan, economic losses due to depression and suicide are estimated to be about 3 trillion yen. If there were no such losses, GDP would increase about 2 trillion yen (an estimate by the Ministry of Health, Labour and Welfare, 2010).
Economic losses around the world are estimated to be about 62 trillion yen in 2002 (Screening for Depression in Adults: A Summary of the Evidence. Ann Intern Med. 2002.). The current economic losses are estimated to be over 100 trillion yen.
◎Treatment of Depression
If someone is diagnosed as having depression, the common treatment is prescription of "antidepressant drugs." The antidepressant drugs can be categorized into several groups such as selective serotonin reuptake inhibitor (SSRI), serotonin-noradrenaline reuptake inhibitor (SNRI), and tricyclic antidepressant. In addition, anti-anxiety drugs or sleep inducing drugs can be used, depending on the symptom.
The important thing under drug therapy is that the patients comply with the prescribed amount and frequency after they are informed about the effects and side effects. However, patients with depression often reduce the amount or frequency without doctors' permission as they do not feel the symptom to be very serious, they worry side effects, etc. In these cases, the patients do not show improvement as doctors expect, and the doctors prescribe more drugs or change the types of drugs. That would often result in the delay of recovery or excessive administration of drugs, for lack of trust between doctors and patients. Therefore, it is essential to have objective assessment standards quantifying the state of depression or proving the depression has been cured. The biomarker and diagnostic agents of the depression that the Company is currently working on are extremely important for prompt and appropriate treatment of the disease.
【Metabolomics and Biomarker】
In order to understand the outline of the Company's business activities, it is necessary to have a certain understanding of two key words, "metabolomics" and "biomarker".
<What is metabolomics?>
Living organisms including human beings consist of some parts with various functions such as muscles, internal organs, and bones. "Metabolites" such as amino acids, fatty acids, and nucleic acids are common and major components of these organs. Metabolites play crucial roles for entire life activities.
Metabolites are provided by food and are consumed in the process of daily actions such as exercise. They move in a body and cells in accordance with their functions and are converted into new substances through various chemical reactions, which are called "metabolism". Adjusting body temperature, breathing, moving heart, digesting and absorbing food, transforming old cells into new ones are all operated by metabolism. The "substance conversion" to a new substance is based on a certain flow called metabolic pathway.
One of well-known approaches to understand the mechanisms of a human body is "genomics", analyzing genes. Now, automated sequencing and computer analysis of genetic information (DNA base sequences) is available, and nearly all the information in the human genome has already been deciphered. However, much about the relationship between the roles of genes and diseases remains unknown. Recently, more researchers lean towards investigating metabolic profiles, in addition to genetic information coming out of genome analysis, in order to understand the relationships between a human body and diseases. Consequently, research and use of "metabolomics (metabolome analysis)" targeting all metabolites is becoming increasingly popular.
The metabolome analysis is mainly used in the following fields:
 <What are biomarkers?>
A human body puts various vital functions under high-sophisticated manipulation to minimize internal and external influences, and subsequently keeps the body condition stable. That mechanism is called "homeostasis". For example, body temperature and heartbeat may change temporarily, but return to regular ranges.
Diseases lead abnormal homeostasis and metabolic status, which are different from healthy conditions. A metabolite, which concentration keenly reflects the status of a specific disease, is called a biomarker. By measuring a biomarker, the current status of a specific disease can be objectively assessed.
Blood sugar as a pancreas function indicator, γ-GTP as a liver function indicator, biomarker PSA for prostatic cancer and biomarker CA19-9 for pancreatic cancer are examples of well-known biomarkers.
Biomarkers have been studied for a long time in order to monitor the status of diseases. These days, with new methods to analyze multiple substances with higher sensitivity all at once, study results of various new biomarkers have been publishing one after another. Among the biomarkers that are explored through metabolomics technologies are the followings: <What are biomarkers?>
A human body puts various vital functions under high-sophisticated manipulation to minimize internal and external influences, and subsequently keeps the body condition stable. That mechanism is called "homeostasis". For example, body temperature and heartbeat may change temporarily, but return to regular ranges.
Diseases lead abnormal homeostasis and metabolic status, which are different from healthy conditions. A metabolite, which concentration keenly reflects the status of a specific disease, is called a biomarker. By measuring a biomarker, the current status of a specific disease can be objectively assessed.
Blood sugar as a pancreas function indicator, γ-GTP as a liver function indicator, biomarker PSA for prostatic cancer and biomarker CA19-9 for pancreatic cancer are examples of well-known biomarkers.
Biomarkers have been studied for a long time in order to monitor the status of diseases. These days, with new methods to analyze multiple substances with higher sensitivity all at once, study results of various new biomarkers have been publishing one after another. Among the biomarkers that are explored through metabolomics technologies are the followings:
 【Business Contents and Business Model】
The main businesses pillars of the Company are the "commissioned metabolome analysis business" and "biomarker business". It is trying to expand the metabolome study related market by disseminating the excellence of its CE-MS system, the Company's core technology, to research institutions and pharmaceutical companies, and securing income base by expanding the metabolome analysis business in Japan and overseas.
Previously, the company allocated the profit from the "metabolome analysis business," which is the current mainstay business, to the investment in the R&D of the promising "biomarker business," and applied the intellectual property obtained through the R&D to the development of medicines and disease diagnosis, with the goal of growing in the mid to long terms. From the term ending Mar. 2017, the company will accelerate the investment in the biomarker business by procuring funds from the outside in order to flourish in the future.
Earnings structure and customers of each business are as follows. 【Business Contents and Business Model】
The main businesses pillars of the Company are the "commissioned metabolome analysis business" and "biomarker business". It is trying to expand the metabolome study related market by disseminating the excellence of its CE-MS system, the Company's core technology, to research institutions and pharmaceutical companies, and securing income base by expanding the metabolome analysis business in Japan and overseas.
Previously, the company allocated the profit from the "metabolome analysis business," which is the current mainstay business, to the investment in the R&D of the promising "biomarker business," and applied the intellectual property obtained through the R&D to the development of medicines and disease diagnosis, with the goal of growing in the mid to long terms. From the term ending Mar. 2017, the company will accelerate the investment in the biomarker business by procuring funds from the outside in order to flourish in the future.
Earnings structure and customers of each business are as follows.
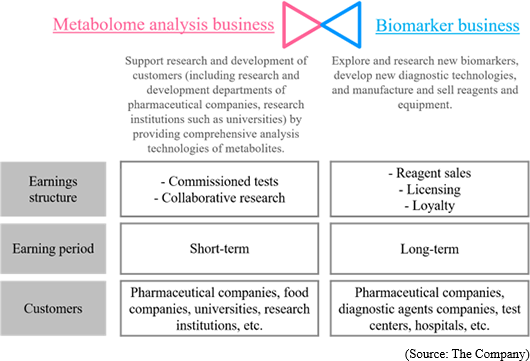 ① Commissioned Metabolome Analysis Business
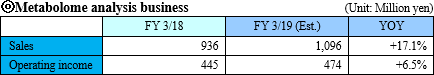
"Sales were 936 million yen and operating income was 445 million yen for FY March 2018."
The Company receives orders from various customers, e.g., private companies such as pharmaceutical companies and food companies as well as universities and public research institutes.
The scheme of the service is as follows. A customer sends samples to the Company, and then metabolites are extracted from the samples. The extracted metabolites are measured by the CE-MS system, and the acquired data is analyzed. After all, the report is delivered to the customer. The data obtained from the service are used for various purposes; basic biological study, assessment of drug effectiveness and toxicity assessment by pharmaceutical companies, universities, and research institutions, analysis of fermentation process and functional evaluation of functional foods by food companies. The data is contributing to the progress of research and development activities of customers. In recent years, not only healthcare, food, but also interest towards health-related companies is emerging rapidly. The Company has not only accumulated a wealth of achievements with a total of 4,750 tests since the foundation up to the fiscal year 2017, but also receives a high reputation from customers for their quality.
◎Deployment in the Overseas Market
In order to distribute the commissioned metabolome analysis service in Asia, the Company concluded an exclusive sales authority agreement with Young In Frontier Co., Ltd. in South Korea in June 2011. Moreover, the Company hired a sales representative in charge of Asia-Pacific area to develop Asian market including Singapore and Hong Kong, outside of South Korea. Furthermore, in order to expand its business in the North American market, it also established its sales subsidiary, Human Metabolome Technologies America, Inc., in October 2012 at Cambridge, Massachusetts, USA, home to many medical research institutions.
In order to further accelerate overseas development, in May 2017, the company established a subsidiary (sub-subsidiary) "Human Metabolome Technologies Europe B.V." in Europe (Netherlands) through HMT-A.
◎"C-SCOPE" -a service package of the commissioned metabolome analysis service for cancer study
In August 2012, the Company launched "C-SCOPE", a service package of the commissioned metabolome analysis service well organized for cancer study. C-SCOPE was developed to respond to the needs; they would like to measure concentrations of specific metabolites changing inside cancer cells with higher sensitivity and higher accuracy. Its technologies are based on unique and efficient metabolites extraction method from cancer cells and highly sensitive analytical system.
Cancer is the number one cause of death in Japan since 1981 and occupies about 30% of the recent total causes of death. According to the MHLW, the costs for cancer research are increasing year by year; in 2012, \35.7 billion were spent. It is an urgent task for many pharmaceutical companies to develop effective new anticancer drugs.
The "Warburg effect" is the phenomenon that most cancer cells have glycolytic rates of several to dozens of times to that of normal cells. Although this effect was proposed as far back as over 80 years ago, the research on the effect made little progress since there was no method to comprehensively measure metabolites back then.
Thanks to the dramatic advancement in metabolomics technologies in recent years, development of anticancer drugs which act as metabolic inhibitors are underway.
The Company's metabolome analysis technology based on the CE-MS system is considered as one of the effective analysis systems applicable at each stage of cancer research; from the basic study of cancer biology to clinical application in the process of anticancer drugs development.
② Biomarker Business
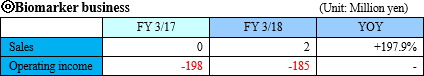
"Sales was 2 million JPY and operating loss was 185 million JPY for FY March 2018"
The Company considers the business related to biomarkers, which play an important role in occasions such as early diagnosis or monitoring treatment effects, as a driver for future growth, and is proceeding with biomarker discovery and development of clinical test drugs through collaborative research and development with universities, pharmaceutical companies, and diagnostic drugs companies.
The Company develops new diagnostic methods by using biomarkers, which were acquired through its own R&D, or biomarkers introduced from outside. Additionally, through the process of product development and clinical development, the Company produces and sells in vivo diagnostic drugs and diagnostic equipment.
The sales in this business are composed of cooperation money for R&D from pharmaceutical companies in cooperative research, milestone revenues, and loyalty gained from the sales of the drugs when they are commercialized.
◎Intellectual Property Policy
The personnel in charge of intellectual property and contracts works on patent application and requests for examination of all projects and are in close collaboration with the patent attorneys of the Company and its collaborative research institution. They are also responsible for negotiation concerning collaboration research agreements and development of agreement documents. The Company attempts to maximize its rights when obtaining a patent of newly discovered biomarkers.
Since the scope of the rights differs depending on the marker, the Company generates patent application documents in a way that would cover a wide scope of the rights such as chemical structure of the biomarker, usage for diagnostics and drug development, detection method and measurement equipment. Furthermore, the Company makes it a principle to file international patent applications in accordance with the Patent Cooperation Treaty, in anticipation for the future license agreement and market based on the information of clinical test drugs, test equipment companies and pharmaceutical companies of various countries.
As of June 2017, the "basic patent" on the method of biomarkers of depression diagnosis etc. has been registered in Japan, the US and China (Europe has been filed). The patent on the method of measuring ethanolamine phosphate (PEA) has been registered at all four stations in Japan, the US, China and Europe.
◎Example of Biomarker Business: Depression Biomarker
The Company especially focuses its research and development on a) the central nervous system disorder such as depression (e.g. mood disorder and mental disorder) for which there are few objective diagnosis methods b) diseases that have become social problems such as metabolic syndrome, including hepatitis and diabetes, and their related diseases. Its current focus is the biomarker for depression.
Diagnosis of the major depressive disorder is conducted using the diagnostic standards provided by the American Psychiatric Association or the standards of the World Health Organization (WHO). However, both of them largely reflect the subjective view of the doctor or patient, and unlike other diseases, no diagnostic method has been established on the basis of objective indicators.
The Company conducted collaborative research with the National Center of Neurology and Psychiatry, and discovered a blood biomarker of the major depressive disorder.
Blood samples were collected from approximately 30 patients and 30 healthy people, and the blood components were compared through metabolome analysis using the CE-MS system. As a result, the patients with major depressive disorder showed lower concentration of (PEA) in their serum.
Through further analysis, it was found that a) PEA is a specific biomarker for major depressive disorder, and b) PEA level will return to the healthy standard range when MDD is treated. ① Commissioned Metabolome Analysis Business
"Sales were 936 million yen and operating income was 445 million yen for FY March 2018."
The Company receives orders from various customers, e.g., private companies such as pharmaceutical companies and food companies as well as universities and public research institutes.
The scheme of the service is as follows. A customer sends samples to the Company, and then metabolites are extracted from the samples. The extracted metabolites are measured by the CE-MS system, and the acquired data is analyzed. After all, the report is delivered to the customer. The data obtained from the service are used for various purposes; basic biological study, assessment of drug effectiveness and toxicity assessment by pharmaceutical companies, universities, and research institutions, analysis of fermentation process and functional evaluation of functional foods by food companies. The data is contributing to the progress of research and development activities of customers. In recent years, not only healthcare, food, but also interest towards health-related companies is emerging rapidly. The Company has not only accumulated a wealth of achievements with a total of 4,750 tests since the foundation up to the fiscal year 2017, but also receives a high reputation from customers for their quality.
◎Deployment in the Overseas Market
In order to distribute the commissioned metabolome analysis service in Asia, the Company concluded an exclusive sales authority agreement with Young In Frontier Co., Ltd. in South Korea in June 2011. Moreover, the Company hired a sales representative in charge of Asia-Pacific area to develop Asian market including Singapore and Hong Kong, outside of South Korea. Furthermore, in order to expand its business in the North American market, it also established its sales subsidiary, Human Metabolome Technologies America, Inc., in October 2012 at Cambridge, Massachusetts, USA, home to many medical research institutions.
In order to further accelerate overseas development, in May 2017, the company established a subsidiary (sub-subsidiary) "Human Metabolome Technologies Europe B.V." in Europe (Netherlands) through HMT-A.
◎"C-SCOPE" -a service package of the commissioned metabolome analysis service for cancer study
In August 2012, the Company launched "C-SCOPE", a service package of the commissioned metabolome analysis service well organized for cancer study. C-SCOPE was developed to respond to the needs; they would like to measure concentrations of specific metabolites changing inside cancer cells with higher sensitivity and higher accuracy. Its technologies are based on unique and efficient metabolites extraction method from cancer cells and highly sensitive analytical system.
Cancer is the number one cause of death in Japan since 1981 and occupies about 30% of the recent total causes of death. According to the MHLW, the costs for cancer research are increasing year by year; in 2012, \35.7 billion were spent. It is an urgent task for many pharmaceutical companies to develop effective new anticancer drugs.
The "Warburg effect" is the phenomenon that most cancer cells have glycolytic rates of several to dozens of times to that of normal cells. Although this effect was proposed as far back as over 80 years ago, the research on the effect made little progress since there was no method to comprehensively measure metabolites back then.
Thanks to the dramatic advancement in metabolomics technologies in recent years, development of anticancer drugs which act as metabolic inhibitors are underway.
The Company's metabolome analysis technology based on the CE-MS system is considered as one of the effective analysis systems applicable at each stage of cancer research; from the basic study of cancer biology to clinical application in the process of anticancer drugs development.
② Biomarker Business
"Sales was 2 million JPY and operating loss was 185 million JPY for FY March 2018"
The Company considers the business related to biomarkers, which play an important role in occasions such as early diagnosis or monitoring treatment effects, as a driver for future growth, and is proceeding with biomarker discovery and development of clinical test drugs through collaborative research and development with universities, pharmaceutical companies, and diagnostic drugs companies.
The Company develops new diagnostic methods by using biomarkers, which were acquired through its own R&D, or biomarkers introduced from outside. Additionally, through the process of product development and clinical development, the Company produces and sells in vivo diagnostic drugs and diagnostic equipment.
The sales in this business are composed of cooperation money for R&D from pharmaceutical companies in cooperative research, milestone revenues, and loyalty gained from the sales of the drugs when they are commercialized.
◎Intellectual Property Policy
The personnel in charge of intellectual property and contracts works on patent application and requests for examination of all projects and are in close collaboration with the patent attorneys of the Company and its collaborative research institution. They are also responsible for negotiation concerning collaboration research agreements and development of agreement documents. The Company attempts to maximize its rights when obtaining a patent of newly discovered biomarkers.
Since the scope of the rights differs depending on the marker, the Company generates patent application documents in a way that would cover a wide scope of the rights such as chemical structure of the biomarker, usage for diagnostics and drug development, detection method and measurement equipment. Furthermore, the Company makes it a principle to file international patent applications in accordance with the Patent Cooperation Treaty, in anticipation for the future license agreement and market based on the information of clinical test drugs, test equipment companies and pharmaceutical companies of various countries.
As of June 2017, the "basic patent" on the method of biomarkers of depression diagnosis etc. has been registered in Japan, the US and China (Europe has been filed). The patent on the method of measuring ethanolamine phosphate (PEA) has been registered at all four stations in Japan, the US, China and Europe.
◎Example of Biomarker Business: Depression Biomarker
The Company especially focuses its research and development on a) the central nervous system disorder such as depression (e.g. mood disorder and mental disorder) for which there are few objective diagnosis methods b) diseases that have become social problems such as metabolic syndrome, including hepatitis and diabetes, and their related diseases. Its current focus is the biomarker for depression.
Diagnosis of the major depressive disorder is conducted using the diagnostic standards provided by the American Psychiatric Association or the standards of the World Health Organization (WHO). However, both of them largely reflect the subjective view of the doctor or patient, and unlike other diseases, no diagnostic method has been established on the basis of objective indicators.
The Company conducted collaborative research with the National Center of Neurology and Psychiatry, and discovered a blood biomarker of the major depressive disorder.
Blood samples were collected from approximately 30 patients and 30 healthy people, and the blood components were compared through metabolome analysis using the CE-MS system. As a result, the patients with major depressive disorder showed lower concentration of (PEA) in their serum.
Through further analysis, it was found that a) PEA is a specific biomarker for major depressive disorder, and b) PEA level will return to the healthy standard range when MDD is treated.
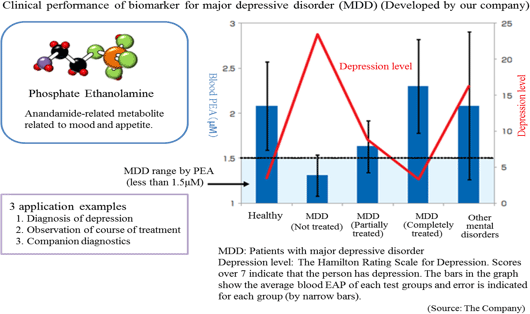 *Companion diagnostics: Refers to clinical tests to predict effects and side effects of pharmaceutical products before administration. By checking the responses of individual patients towards a drug before treatment, more effective drug administration can be provided.
As described earlier, the Company explores, studies and analyzes the mechanisms of PEA and depression. Meanwhile, they developed an instrumental analysis measurement method, which improved the Company's measurement accuracy and enabled them to study treatment effects and condition of diseases.
Subsequently, the Company developed a technology to measure extremely low-concentrated PEA in the blood, which was not possible to detect with the conventional enzyme method.
Based on this technology, the Company developed the "PEA test kit using the enzyme method" (β-version) in 2016.
It is of extreme significance that the Company succeeded in the development of a "depression biomarker test kit" (β-version).
"Realization of an inspection method that can be processed cheaply and massively" and "Establishment of a technical foundation that can supply reagent kits worldwide" will make it possible to supply PEA tests to 350 million people with depression worldwide. As a result, the company's significance of social existence has become even bigger. In addition, it has become possible to shift to a new business development phase that considers concrete markets, product specifications, distribution channel construction, business size, etc.
(Measurement reagent kit - For research use -) *Companion diagnostics: Refers to clinical tests to predict effects and side effects of pharmaceutical products before administration. By checking the responses of individual patients towards a drug before treatment, more effective drug administration can be provided.
As described earlier, the Company explores, studies and analyzes the mechanisms of PEA and depression. Meanwhile, they developed an instrumental analysis measurement method, which improved the Company's measurement accuracy and enabled them to study treatment effects and condition of diseases.
Subsequently, the Company developed a technology to measure extremely low-concentrated PEA in the blood, which was not possible to detect with the conventional enzyme method.
Based on this technology, the Company developed the "PEA test kit using the enzyme method" (β-version) in 2016.
It is of extreme significance that the Company succeeded in the development of a "depression biomarker test kit" (β-version).
"Realization of an inspection method that can be processed cheaply and massively" and "Establishment of a technical foundation that can supply reagent kits worldwide" will make it possible to supply PEA tests to 350 million people with depression worldwide. As a result, the company's significance of social existence has become even bigger. In addition, it has become possible to shift to a new business development phase that considers concrete markets, product specifications, distribution channel construction, business size, etc.
(Measurement reagent kit - For research use -)
 ◎Identification of Disease Biomarkers
The Company uses the following three connections and systems for identifying biomarkers in order to expand biomarker development pipelines.
<Connection with the Customers for Commissioned Analysis or Collaborative Development>
The Company accepts requests from universities and companies for the tests for finding biomarkers. The Company also receives proposals for collaborative development before or after the tests. Currently, a collaborative development project for diabetic nephropathy biomarkers is ongoing.
<Direct Proposal to Researchers and Physicians>
The Company's researchers directly propose research plans for the development of disease biomarkers to researchers or physicians, and establish collaborative study agreement with the institution based on approvals from the collaborative researchers or physicians. The target diseases are chosen according to the number of patients, compatibility to the analysis technologies of the Company, degree of social contribution, and necessity of biomarkers. In addition to major depressive disorder, the Company is working on the development of biomarkers for non-alcoholic steatohepatitis and fibromyalgia.
<HMT Research Grant for Young Leaders in Metabolomics>
The Company offers a grant (HMT Research Grant for Young Leaders in Metabolomics) to graduate students to disseminate the usefulness of metabolomics in the society and nurture young researchers. From the research themes submitted from graduate students across the world, the Company chooses excellent proposals, and supports their research by awarding metabolome analysis service without a fee. Fourteen students have been awarded in the last 4 years. Some of these study results actually led to the identification of biomarkers and evolved to collaborative study with the Company, for example, infectious disease-related encephalopathy biomarker. ◎Identification of Disease Biomarkers
The Company uses the following three connections and systems for identifying biomarkers in order to expand biomarker development pipelines.
<Connection with the Customers for Commissioned Analysis or Collaborative Development>
The Company accepts requests from universities and companies for the tests for finding biomarkers. The Company also receives proposals for collaborative development before or after the tests. Currently, a collaborative development project for diabetic nephropathy biomarkers is ongoing.
<Direct Proposal to Researchers and Physicians>
The Company's researchers directly propose research plans for the development of disease biomarkers to researchers or physicians, and establish collaborative study agreement with the institution based on approvals from the collaborative researchers or physicians. The target diseases are chosen according to the number of patients, compatibility to the analysis technologies of the Company, degree of social contribution, and necessity of biomarkers. In addition to major depressive disorder, the Company is working on the development of biomarkers for non-alcoholic steatohepatitis and fibromyalgia.
<HMT Research Grant for Young Leaders in Metabolomics>
The Company offers a grant (HMT Research Grant for Young Leaders in Metabolomics) to graduate students to disseminate the usefulness of metabolomics in the society and nurture young researchers. From the research themes submitted from graduate students across the world, the Company chooses excellent proposals, and supports their research by awarding metabolome analysis service without a fee. Fourteen students have been awarded in the last 4 years. Some of these study results actually led to the identification of biomarkers and evolved to collaborative study with the Company, for example, infectious disease-related encephalopathy biomarker.
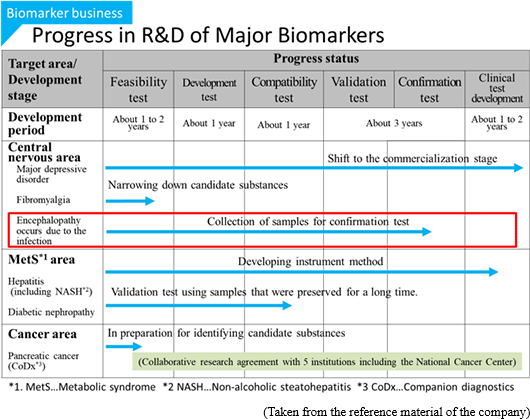 In March 2017, the company announced that it acquired a technology patent concerning the next generation biomarker. Currently it is difficult to detect with conventional CE - MS metabolome analysis technology because γ - glutamyl dipeptide, which is a biomarker for liver diseases currently under development at the company, exists in trace amounts in vivo, but using this patented technique , it is now possible to comprehensively detect γ - glutamyl dipeptides. According to this patented technology, it is possible to realize highly sensitive detection of 50 times or more depending on the substance, therefore it is possible to dramatically improve CE-MS metabolome analysis technology of the company, and the possibility of discovering new innovative biomarkers is also expected to increase.
Furthermore, in Oct. 2017, the "method for detecting encephalopathy" was patented in Japan, as the company and Nagoya University jointly applied for the patent. Encephalopathy occurs due to the infection with viruses, bacteria, or the like, and induces the impairment of consciousness, spasm, abnormal behavior, hallucination, etc. Especially, acute encephalopathy, such as influenza-associated encephalopathy and HHV-6 encephalopathy, occurs to children, and in many cases, it progresses rapidly and becomes severe. Accordingly, it is necessary to diagnoze them early and offer appropriate treatment immediately. In collaborative research with Nagoya University, the company discovered a candidate biomarker for detecting acute encephalopathy, and made it patented.
In March 2017, the company announced that it acquired a technology patent concerning the next generation biomarker. Currently it is difficult to detect with conventional CE - MS metabolome analysis technology because γ - glutamyl dipeptide, which is a biomarker for liver diseases currently under development at the company, exists in trace amounts in vivo, but using this patented technique , it is now possible to comprehensively detect γ - glutamyl dipeptides. According to this patented technology, it is possible to realize highly sensitive detection of 50 times or more depending on the substance, therefore it is possible to dramatically improve CE-MS metabolome analysis technology of the company, and the possibility of discovering new innovative biomarkers is also expected to increase.
Furthermore, in Oct. 2017, the "method for detecting encephalopathy" was patented in Japan, as the company and Nagoya University jointly applied for the patent. Encephalopathy occurs due to the infection with viruses, bacteria, or the like, and induces the impairment of consciousness, spasm, abnormal behavior, hallucination, etc. Especially, acute encephalopathy, such as influenza-associated encephalopathy and HHV-6 encephalopathy, occurs to children, and in many cases, it progresses rapidly and becomes severe. Accordingly, it is necessary to diagnoze them early and offer appropriate treatment immediately. In collaborative research with Nagoya University, the company discovered a candidate biomarker for detecting acute encephalopathy, and made it patented.
|
| Fiscal Year March 2018 Earnings Results |
|
(1) Consolidated Business Results
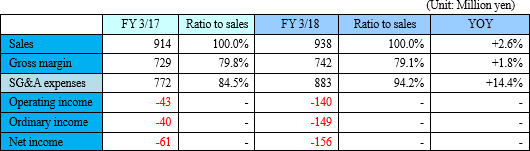 Loss continues due to increasing investment for growth.
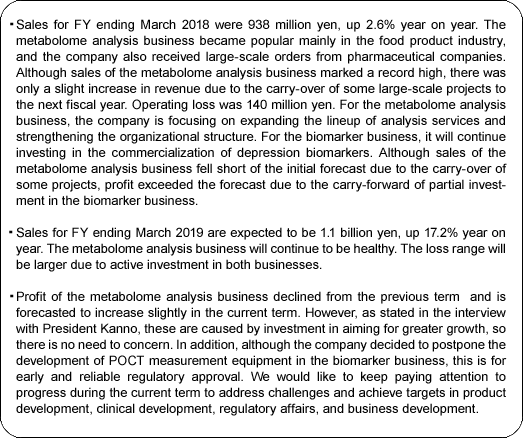
Sales were 938 million yen, up 2.6% year on year. The metabolome analysis business became popular mainly in the food product industry, and the company also received large-scale orders from pharmaceutical companies. Although sales of the metabolome analysis business marked a record high, there was only a slight increase in revenue due to the carry-over of some large-scale projects to the next fiscal year.
Operating loss was 140 million yen. For the metabolome analysis business, the company is focusing on expanding the lineup of analysis services and strengthening the organizational structure. For the biomarker business, it will continue investing in the commercialization of depression biomarkers.
Although sales of the metabolome analysis business fell short of the initial forecast due to the carry-over of some projects, profit exceeded the forecast due to the carry-forward of partial investment in the biomarker business.
(2) Highlights by Major Segment Loss continues due to increasing investment for growth.
Sales were 938 million yen, up 2.6% year on year. The metabolome analysis business became popular mainly in the food product industry, and the company also received large-scale orders from pharmaceutical companies. Although sales of the metabolome analysis business marked a record high, there was only a slight increase in revenue due to the carry-over of some large-scale projects to the next fiscal year.
Operating loss was 140 million yen. For the metabolome analysis business, the company is focusing on expanding the lineup of analysis services and strengthening the organizational structure. For the biomarker business, it will continue investing in the commercialization of depression biomarkers.
Although sales of the metabolome analysis business fell short of the initial forecast due to the carry-over of some projects, profit exceeded the forecast due to the carry-forward of partial investment in the biomarker business.
(2) Highlights by Major Segment
 Sales were 936 million yen, up 2.5%. Orders in Japan were strong because of development in the food product industry and large-scale projects from pharmaceutical companies. As for overseas, orders also increased in the pharmaceuticals and clinical fields mainly in the United States. However, due to the carry-over of some large projects to the next fiscal year, there was only a slight increase in revenue. Profit decreased 11.2% year on year, as the company expanded the lineup of analysis service and strengthened the organization structure.
Orders received and order backlogs were strong, up 8.9% and 62.6% year on year, respectively.
Sales were 936 million yen, up 2.5%. Orders in Japan were strong because of development in the food product industry and large-scale projects from pharmaceutical companies. As for overseas, orders also increased in the pharmaceuticals and clinical fields mainly in the United States. However, due to the carry-over of some large projects to the next fiscal year, there was only a slight increase in revenue. Profit decreased 11.2% year on year, as the company expanded the lineup of analysis service and strengthened the organization structure.
Orders received and order backlogs were strong, up 8.9% and 62.6% year on year, respectively.
 (3) Financial Condition and Cash Flow (3) Financial Condition and Cash Flow
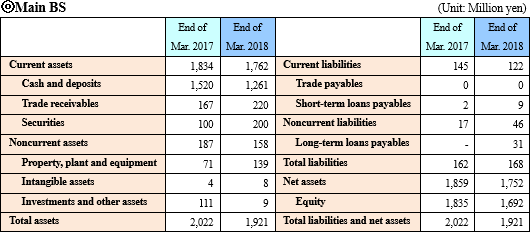 Current assets declined 71 million yen from the end of the previous term, due to decrease in cash and deposits, etc. Noncurrent assets decreased 29 million yen due to a reduction in investment securities, and total assets dropped 100 million yen from the end of the previous term to 1,921 million yen. Total liabilities are nearly unchanged. As the deficit of retained earnings augmented, net assets decreased 106 million yen from the end of the previous term to 1,752 million yen.
As a result, equity ratio declined 2.2 points from 91.4% at the end of the previous term to 89.2%.
Current assets declined 71 million yen from the end of the previous term, due to decrease in cash and deposits, etc. Noncurrent assets decreased 29 million yen due to a reduction in investment securities, and total assets dropped 100 million yen from the end of the previous term to 1,921 million yen. Total liabilities are nearly unchanged. As the deficit of retained earnings augmented, net assets decreased 106 million yen from the end of the previous term to 1,752 million yen.
As a result, equity ratio declined 2.2 points from 91.4% at the end of the previous term to 89.2%.
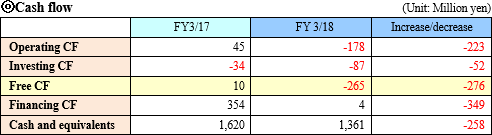 Operating CF turned negative due to the expansion of deficit amount. The deficit of investing CF increased due to the purchase of property, plant and equipment. Free CF also became negative. The positive amount of financing CF became smaller because the proceeds from the issuance of shares resulting from the exercise of subscription rights to shares decreased. The cash position degraded.
Operating CF turned negative due to the expansion of deficit amount. The deficit of investing CF increased due to the purchase of property, plant and equipment. Free CF also became negative. The positive amount of financing CF became smaller because the proceeds from the issuance of shares resulting from the exercise of subscription rights to shares decreased. The cash position degraded.
|
| Fiscal Year March 2019 Earnings Estimates |
|
(1) Consolidated Business Forecasts
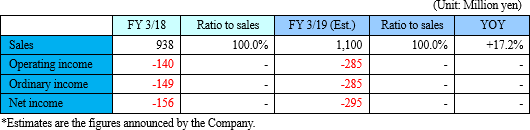 The metabolome analysis business will continue to be strong, and sales will increase. Due to upfront investment for both businesses, the amount of loss will increase.
Sales are forecasted to be 1.1 billion yen, up 17.2% year on year. The metabolome analysis business continued to be healthy. The loss range will be larger due to active investment in both businesses.
(2) Efforts of this term
The company lists the following 4 management policies.
① Sustainable growth of sales and attainment of a budget.
The company will create a foundation for medium-term leap by exploiting new fields and areas by new products and developing new businesses.
② Equipment investment in the analysis business
The company will make a capital investment corresponding to new product development and double the analysis/ production capacity in the medium term.
③ To ensure commercialization of depression biomarker
The company will develop an exit strategy to accelerate clinical research and obtain pharmaceutical approval of the PEA reagent kit.
④ To continue securing stable shareholders and maintaining them via dialogues.
The company will continue focusing on IR activities for institutional investors and individual investors. The metabolome analysis business will continue to be strong, and sales will increase. Due to upfront investment for both businesses, the amount of loss will increase.
Sales are forecasted to be 1.1 billion yen, up 17.2% year on year. The metabolome analysis business continued to be healthy. The loss range will be larger due to active investment in both businesses.
(2) Efforts of this term
The company lists the following 4 management policies.
① Sustainable growth of sales and attainment of a budget.
The company will create a foundation for medium-term leap by exploiting new fields and areas by new products and developing new businesses.
② Equipment investment in the analysis business
The company will make a capital investment corresponding to new product development and double the analysis/ production capacity in the medium term.
③ To ensure commercialization of depression biomarker
The company will develop an exit strategy to accelerate clinical research and obtain pharmaceutical approval of the PEA reagent kit.
④ To continue securing stable shareholders and maintaining them via dialogues.
The company will continue focusing on IR activities for institutional investors and individual investors.
 (External environment)
Metabolomics has advanced from traditional academic technology for universities and laboratories to industrial technology.
Under these circumstances, health-conscious markets with keywords such as new health food products including functional foods, sports, food, sleep, and stress are interested in the usefulness of metabolomics to grasp human health status, and new markets are being created. In addition, the drug development field is starting to pay attention to the benefit of using metabolomics for research on intestinal bacteria, early detection, diagnosis and development of therapy for psychoneurotic diseases such as dementia and Alzheimer's disease, and practical application of medical technology including drugs for refractory diseases.
(Key measures)
In the following wind, the company is planning to undertake the measures below and steadily expand the businesses.
(External environment)
Metabolomics has advanced from traditional academic technology for universities and laboratories to industrial technology.
Under these circumstances, health-conscious markets with keywords such as new health food products including functional foods, sports, food, sleep, and stress are interested in the usefulness of metabolomics to grasp human health status, and new markets are being created. In addition, the drug development field is starting to pay attention to the benefit of using metabolomics for research on intestinal bacteria, early detection, diagnosis and development of therapy for psychoneurotic diseases such as dementia and Alzheimer's disease, and practical application of medical technology including drugs for refractory diseases.
(Key measures)
In the following wind, the company is planning to undertake the measures below and steadily expand the businesses.
 (Points of each measure)
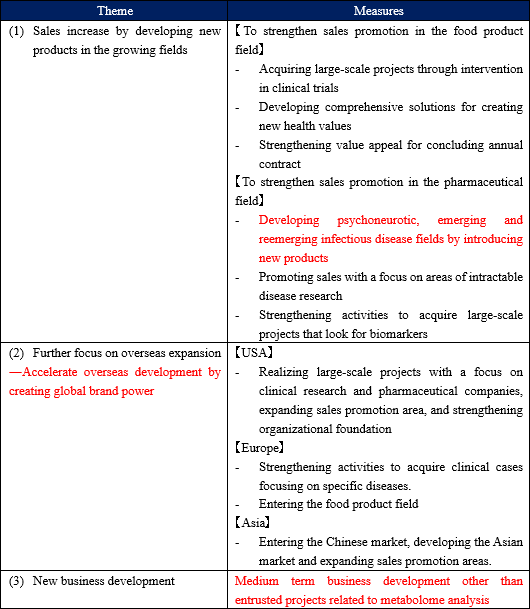
"Sales increase by developing new products in the growing fields: Developing psychoneurotic, emerging and reemerging infectious disease fields by introducing new products" (Points of each measure)
"Sales increase by developing new products in the growing fields: Developing psychoneurotic, emerging and reemerging infectious disease fields by introducing new products"
 Launch of a new service, "Mediator-Scan"
Mediator Scan is the best plan for the purpose of elucidating the pathology and exploring biomarkers of various diseases that are related to immunity and inflammation such as asthma, urticaria, rheumatism, inflammatory bowel disease, atopy, and food allergy as well as lifestyle diseases such as diabetes and arteriosclerosis, and also cancer and dementia. By combining with the company's other entrusted analysis service, it becomes possible to further enhance analysis coverage. Launch of a new service, "Mediator-Scan"
Mediator Scan is the best plan for the purpose of elucidating the pathology and exploring biomarkers of various diseases that are related to immunity and inflammation such as asthma, urticaria, rheumatism, inflammatory bowel disease, atopy, and food allergy as well as lifestyle diseases such as diabetes and arteriosclerosis, and also cancer and dementia. By combining with the company's other entrusted analysis service, it becomes possible to further enhance analysis coverage.
 Launch of a new plan for its unique platform CE-OrbitrapMS within the fiscal year
Orbitrap mass spectrometer (Orbitrap MS) is characterized by high resolution capability and stable mass accuracy, and, in general, it can be used in a wide range of application fields including drug metabolism, impurity analysis, natural products, metabolomics and proteomics. Compared with the conventional CE-TOFMS method, the sensitivity is several times to tens of times better, and the number of detected substances increases about two-fold according to the company.
Users can collectively detect metabolic substances whose concentrations in their living bodies are extremely low. Furthermore, because of the increase in the number of detection substances, there is a high possibility of detecting completely new biomarkers that cannot be found by conventional techniques and services of other companies.
Since it is a unique platform of the company, it is expecting that it will contribute to the expansion of the domestic market, European and US markets as well as acceleration of the business expansion in China.
"Further focus on overseas expansion: Accelerate overseas development by creating global brand power"
The company currently provides metabolome analysis services through its subsidiaries in the US and Europe, and, in Asia, it is operating the business through agencies and from Japan. In addition to this, it will start full-scale development of the Chinese market during this fiscal year.
Although the amount of investment in research and development (R&D) in China is less than that of the US, it exceeds that of Japan in the life science field, and it carries out R&D on a scale comparable to that of Europe. The number of papers published by Chinese research institutions is about four times that of Japan and is outpacing the leading US. Furthermore, in terms of fields, it is the second largest in life sciences in Asia. In addition to the scale, the level is also high. The company considers that the needs for its metabolome analysis technology are high, so the company will accelerate overseas development of the business in collaboration with US and European subsidiaries.
An important weapon at that time is positioned as "the original platform CE-Orbitrap MS" mentioned above.
"New business development: Medium-term business development other than entrusted projects related to metabolome analysis"
As mentioned above, the health-conscious market is focusing on the utility of metabolomics to understand human health status, and new markets are being created. The company expects that the needs for the healthcare industry will be enhanced to clarify and examine mechanisms of dietary effects and exercise effects, and to accurately grasp and judge comfortable sleeping states and stress levels.. Based on the dominant overwhelming achievement in the domestic market of 4,750 cumulative total number of tests up to the year 2017, the company is working on "health marker to detect health", "marker to discover state of mind" , "Markers that contribute to the prevention of disease" and so on. Launch of a new plan for its unique platform CE-OrbitrapMS within the fiscal year
Orbitrap mass spectrometer (Orbitrap MS) is characterized by high resolution capability and stable mass accuracy, and, in general, it can be used in a wide range of application fields including drug metabolism, impurity analysis, natural products, metabolomics and proteomics. Compared with the conventional CE-TOFMS method, the sensitivity is several times to tens of times better, and the number of detected substances increases about two-fold according to the company.
Users can collectively detect metabolic substances whose concentrations in their living bodies are extremely low. Furthermore, because of the increase in the number of detection substances, there is a high possibility of detecting completely new biomarkers that cannot be found by conventional techniques and services of other companies.
Since it is a unique platform of the company, it is expecting that it will contribute to the expansion of the domestic market, European and US markets as well as acceleration of the business expansion in China.
"Further focus on overseas expansion: Accelerate overseas development by creating global brand power"
The company currently provides metabolome analysis services through its subsidiaries in the US and Europe, and, in Asia, it is operating the business through agencies and from Japan. In addition to this, it will start full-scale development of the Chinese market during this fiscal year.
Although the amount of investment in research and development (R&D) in China is less than that of the US, it exceeds that of Japan in the life science field, and it carries out R&D on a scale comparable to that of Europe. The number of papers published by Chinese research institutions is about four times that of Japan and is outpacing the leading US. Furthermore, in terms of fields, it is the second largest in life sciences in Asia. In addition to the scale, the level is also high. The company considers that the needs for its metabolome analysis technology are high, so the company will accelerate overseas development of the business in collaboration with US and European subsidiaries.
An important weapon at that time is positioned as "the original platform CE-Orbitrap MS" mentioned above.
"New business development: Medium-term business development other than entrusted projects related to metabolome analysis"
As mentioned above, the health-conscious market is focusing on the utility of metabolomics to understand human health status, and new markets are being created. The company expects that the needs for the healthcare industry will be enhanced to clarify and examine mechanisms of dietary effects and exercise effects, and to accurately grasp and judge comfortable sleeping states and stress levels.. Based on the dominant overwhelming achievement in the domestic market of 4,750 cumulative total number of tests up to the year 2017, the company is working on "health marker to detect health", "marker to discover state of mind" , "Markers that contribute to the prevention of disease" and so on.
 (3) Topics
◎Introduction of the Executive Officer System
In order to respond appropriately and promptly to changes in the business environment surrounding, to strengthen corporate governance by separating supervisory functions and business execution functions in management, and to clarify responsibility for executing business, as of July 1, 2018, the company introduced the executive officer system. Five current board directors as well as two newly invited people will be appointed as executive officers (two board directors will leave the director position and become the executive officers).
One of them, Mr. Katsuhito Hashizume, was responsible for business development and sales at Takara Bio Inc., the top biotechnology company in Japan. The other one, Mr. Yuji Suzuki, moved from the Ministry of Foreign Affairs to the major consulting company McKinsey & Company Japan, and thereafter, he was in charge of management strategy and global development at Macromill, Inc.
The company is expecting them to be the drivers for the further leap. (3) Topics
◎Introduction of the Executive Officer System
In order to respond appropriately and promptly to changes in the business environment surrounding, to strengthen corporate governance by separating supervisory functions and business execution functions in management, and to clarify responsibility for executing business, as of July 1, 2018, the company introduced the executive officer system. Five current board directors as well as two newly invited people will be appointed as executive officers (two board directors will leave the director position and become the executive officers).
One of them, Mr. Katsuhito Hashizume, was responsible for business development and sales at Takara Bio Inc., the top biotechnology company in Japan. The other one, Mr. Yuji Suzuki, moved from the Ministry of Foreign Affairs to the major consulting company McKinsey & Company Japan, and thereafter, he was in charge of management strategy and global development at Macromill, Inc.
The company is expecting them to be the drivers for the further leap.
|
| Progress of the Biomarker Business |
|
As a result of the development of "PEA test kit using the enzyme method," the company will be able to supply PEA tests to 350 million patients with depression all over the world. It is now steadily developing a foothold for commercialization.
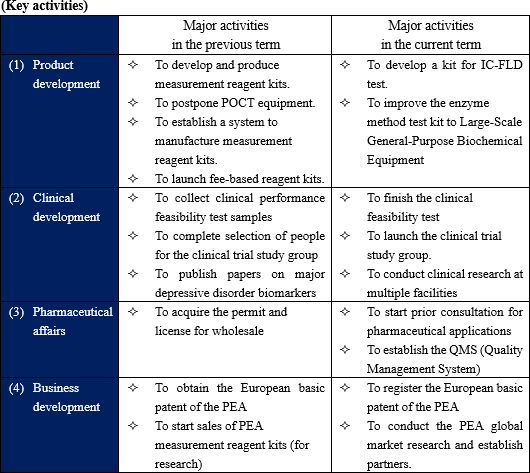 (1) Product development
As for the enzyme method reagent kit for PEA test (for research), which is under development, through a collaborative research with Toyobo Co., Ltd. (3101, the first section of TSE), which is the second largest share of industrial enzymes in the world, the company established mass production technology of enzymes used for measuring depression-related biomarkers and established a system capable of improved and stable supply.
It is working on establishing manufacturing and sales systems of reagents and customer correspondence system and preparing for large-scale sales. At the same time, it began fee-based shipment in the previous fiscal year. During this fiscal year, it will start offering fee-based shipment to research institutes and collecting customers' opinions.
The company planned to develop two types of PEA measurement: a) detailed testing with versatile biochemical instruments at large scale hospitals or clinical test institutes and b) rapid testing with a specialized small POCT bench-top machine and kit at small internal medicine or mental clinics. However, for the plan b), since it needs more time for development of the POCT machine and kit for clinical testing by many co-research institutes, and the company considered it would be more appropriate to forward an pharmaceutical application with the current ongoing IC-FLD (ion-chromatography) method kit firstly rather than the POCT package, and make an aggressive investment to the optimization of the enzymatic reagent kit for large-scale instruments and implementation of clinical studies in order to obtain pharmaceutical regulatory approval as soon as possible.
(2) Clinical development
The progress of clinical research and academic research indispensable for obtaining licenses as in-vitro diagnostic drugs is as follows.
(Clinical development) (1) Product development
As for the enzyme method reagent kit for PEA test (for research), which is under development, through a collaborative research with Toyobo Co., Ltd. (3101, the first section of TSE), which is the second largest share of industrial enzymes in the world, the company established mass production technology of enzymes used for measuring depression-related biomarkers and established a system capable of improved and stable supply.
It is working on establishing manufacturing and sales systems of reagents and customer correspondence system and preparing for large-scale sales. At the same time, it began fee-based shipment in the previous fiscal year. During this fiscal year, it will start offering fee-based shipment to research institutes and collecting customers' opinions.
The company planned to develop two types of PEA measurement: a) detailed testing with versatile biochemical instruments at large scale hospitals or clinical test institutes and b) rapid testing with a specialized small POCT bench-top machine and kit at small internal medicine or mental clinics. However, for the plan b), since it needs more time for development of the POCT machine and kit for clinical testing by many co-research institutes, and the company considered it would be more appropriate to forward an pharmaceutical application with the current ongoing IC-FLD (ion-chromatography) method kit firstly rather than the POCT package, and make an aggressive investment to the optimization of the enzymatic reagent kit for large-scale instruments and implementation of clinical studies in order to obtain pharmaceutical regulatory approval as soon as possible.
(2) Clinical development
The progress of clinical research and academic research indispensable for obtaining licenses as in-vitro diagnostic drugs is as follows.
(Clinical development)
 Concerning the performance test of plasma PEA by a case-control study, the company-led feasibility test is in progress. Concerning the performance test of plasma PEA by a case-control study, the company-led feasibility test is in progress.
 The company is in the process of scheduling collaborative research with domestic university hospitals and US university hospitals on verification of relevant diseases, genetic background and racial differences by domestic and foreign cohorts. The company is in the process of scheduling collaborative research with domestic university hospitals and US university hospitals on verification of relevant diseases, genetic background and racial differences by domestic and foreign cohorts.
 As for preparation for domestic clinical trials, a partial contract was completed to start working collaboratively with domestic university hospitals.
(Academic research) As for preparation for domestic clinical trials, a partial contract was completed to start working collaboratively with domestic university hospitals.
(Academic research)
 For elucidation of the decreasing plasma PEA mechanism by depression model animals, joint research with domestic universities was conducted, and a decrease of plasma PEA in the model animals was confirmed. For elucidation of the decreasing plasma PEA mechanism by depression model animals, joint research with domestic universities was conducted, and a decrease of plasma PEA in the model animals was confirmed.
 Joint research with domestic universities is underway to verify the effect of antidepressant administration on plasma PEA concentration among model animals. Joint research with domestic universities is underway to verify the effect of antidepressant administration on plasma PEA concentration among model animals.
 The company is conducting joint research with US National Institute of Health on the elucidation of PEA generation mechanism in brain using biochemical methods.
In January 2018, an article on the major depressive disorder biomarker was published in the journal of the Japan Society of Psychiatry and Neurology. According to this article, metabolome analysis comparing the blood components between major depressive disorder patients and healthy volunteers revealed a metabolome profile characterizing the major depressive disorder patients with PEA as a biomarker.
(Summary) The company is conducting joint research with US National Institute of Health on the elucidation of PEA generation mechanism in brain using biochemical methods.
In January 2018, an article on the major depressive disorder biomarker was published in the journal of the Japan Society of Psychiatry and Neurology. According to this article, metabolome analysis comparing the blood components between major depressive disorder patients and healthy volunteers revealed a metabolome profile characterizing the major depressive disorder patients with PEA as a biomarker.
(Summary)
 The metabolome analysis of the patients with major depressive disorder and healthy subjects by the CE-MS method, which is the company's core technology, revealed that plasma PEA among the patients was significantly lower than that of healthy subjects. The metabolome analysis of the patients with major depressive disorder and healthy subjects by the CE-MS method, which is the company's core technology, revealed that plasma PEA among the patients was significantly lower than that of healthy subjects.
 As a result of the measurement of plasma PEA of the patients with and without major depressive disorder by the ion chromatography-fluorescence detection (IC-FLD) method, plasma PEA showed high discrimination performance as a biomarker of the major depressive disorder patients. As a result of the measurement of plasma PEA of the patients with and without major depressive disorder by the ion chromatography-fluorescence detection (IC-FLD) method, plasma PEA showed high discrimination performance as a biomarker of the major depressive disorder patients.
 Comparison of the PEA between the patients with and without major depressive disorder was carried out as a verification test. The results showed that the plasma PEA concentration was a promising biomarker candidate for major depressive disorder.
(3) Pharmaceutical affairs
In addition to obtaining the permission for the manufacture and sale of pharmaceutical products for in-vitro diagnosis in the fiscal year before last, the company obtained the permission for "wholesale distribution of pharmaceutical products" in the previous fiscal year.
As the company managed to advance the development of an important foundation for pharmaceutical business for the future commercialization, it will begin prior consultation for pharmaceutical applications with Pharmaceuticals and Medical Devices Agency during this fiscal year and will begin discussing specific exit strategies.
(4) Business development
As mentioned above, the company started offering fee-based measurement reagent kits (for research) in the previous fiscal year.
The basic patent of the PEA enzymatic method is not yet registered in Europe, but it is expected to be registered soon. Comparison of the PEA between the patients with and without major depressive disorder was carried out as a verification test. The results showed that the plasma PEA concentration was a promising biomarker candidate for major depressive disorder.
(3) Pharmaceutical affairs
In addition to obtaining the permission for the manufacture and sale of pharmaceutical products for in-vitro diagnosis in the fiscal year before last, the company obtained the permission for "wholesale distribution of pharmaceutical products" in the previous fiscal year.
As the company managed to advance the development of an important foundation for pharmaceutical business for the future commercialization, it will begin prior consultation for pharmaceutical applications with Pharmaceuticals and Medical Devices Agency during this fiscal year and will begin discussing specific exit strategies.
(4) Business development
As mentioned above, the company started offering fee-based measurement reagent kits (for research) in the previous fiscal year.
The basic patent of the PEA enzymatic method is not yet registered in Europe, but it is expected to be registered soon.
|
| Interview with President Kanno |
|
We asked President Kanno about the current situation and prospects of both businesses and asked him to give messages to the shareholders and investors.
Q: "What are the points and topics of both businesses in the previous term?"
A: "Orders for the metabolome analysis business exceeded 1 billion yen for the first time. For the biomarker business, the most notable point is that an article that objectively recognized the usefulness of PEA as a biomarker of major depressive disorder was published."
The metabolome analysis business exceeded 1 billion yen for the first time.
We believe that the major factors for this success were our efforts to acquire large orders as well as an increase in use of the metabolome analysis technology, which has been mainly used by academia such as universities and laboratories, by private enterprises, mainly food companies that have high interest in "quality control" and "proof of value" in recent years. This means that the social utilization of value of metabolome analysis is becoming clearer. Having said so, sales did not reach 1 billion yen due to the carry-over of some of the projects to the next fiscal year, even though orders exceeded 1 billion yen.
We believe that a more appropriate order management system is necessary.
The biggest topic of the biomarker business is that, in January 2018, a paper on the major depressive disorder biomarker was published in the journal "Psychiatry and Clinical Neurosciences" of the Japan Society of Psychiatry and Neurology.
We hope that the usefulness of PEA as a biomarker of major depressive disorder will be objectively recognized again and the recognition will be spread further.
Q: "Please tell us your future points of focus in the metabolome analysis business."
A: "One is the development of new products and new services. We will devote ourselves to exploring overseas markets where our brand power is still low, taking advantage of the overwhelming performance of our unique platform in metabolome analysis. Another point is the development of new businesses other than the entrusted projects. We will effectively utilize a wide range of about 5,000 "Big Data," which are our important assets, and will establish completely new businesses."
Profit of the metabolome analysis business decreased in the previous fiscal year. The profit growth rate also remains only one digit during this fiscal year. This is because we are making various investments in the metabolome analysis business which is expected to grow significantly, although it is our basic route to make stable revenue in the metabolome analysis business and grow significantly through monetization of the biomarker business. Therefore, it does not mean that our profitability has deteriorated.
We are focusing our investments in 2 fields.
One is the development of new products and new services.
The high sensitivity of CE-OrbitrapMS, our original platform, enables to detect substances that could not be detected by conventional methods. This technical advantage creates an advantage of reducing the number of samples and also enhances the possibility of detecting completely new biomarkers which could not be found up to now.
We are the only one that offers this technology in the world, and we will work on the development of overseas markets where our brand power is still low, using the overwhelming performance difference as a weapon.
Another field, which is our medium-term initiative, is the development of new businesses other than entrusted projects related to metabolome analysis.
For example, by effectively utilizing our wide range of about 5,000 "Big Data," which are important assets of our company, we will establish a new business to develop "health markers" and provide them as information not in the medical field but in the healthcare field.
Q: "How about the biomarker business?"
A: "We decided that it would be appropriate to work on equipment development targeting clinical laboratory centers and clinical laboratories in large hospitals to obtain early and reliable pharmaceutical regulatory approvals. Meanwhile, the possibility of creating more demand in examination fields other than depression diagnosis is expanding, and we aim to expand sales scale by various measures."
Some trajectory corrections were made. Initiatives are progressing steadily in the fields of product development, clinical development, pharmaceutical affairs, and business development. However, in order to obtain early and reliable pharmaceutical regulatory approvals, the development of POCT measurement equipment should be postponed, and we should focus on the pharmaceutical regulatory application of the IC-FLD method test kit targeting clinical laboratory centers and clinical laboratories in large hospitals and improvement of the enzyme reagent kit for large-scale biochemical examination apparatus.
Meanwhile, there is a possibility that more demand can be created by promoting approaches and mechanism development for the examination fields other than depression diagnosis. We aim to expand sales scale by further investigating the market size, constructing sales channels, and setting appropriate sales prices.
Q: "In the end, please give messages to the shareholders and investors."
A: "We have entered a new phase of three years. We consider these years as a phase to fruit the results of investments we made so far for both businesses. We would like to ask you to support us as we make concerted efforts to respond to the expectations of shareholders and investors."
Three years have passed since we got listed, and we have started a new three-year plan during this fiscal year. We consider this period for both businesses to bear fruit of our past investment.
While the metabolome analysis business is steadily up-taking domestic demand, we will develop the global market with our unique technology.
For the biomarker business, we have made some trajectory corrections. These were done for early and reliable pharmaceutical regulatory approvals, and we have entered an important stage.
In addition, we acquired new strengths as we introduced the executive officer system across the entire company, and we can expect stronger management and promotion of our businesses.
We would like to ask for your support, as we make concerted efforts to respond to the expectations of our shareholders and investors.
|
| Conclusions |
|
Profit of the metabolome analysis business decreased in the previous term and is forecasted to increase slightly in the current term. However, as stated in the interview with President Kanno, these are caused by investment in aiming for greater growth, so there is no need to concern. In addition, although the company decided to postpone the development of POCT measurement equipment in the biomarker business, this is for early and reliable regulatory approval. We would like to keep paying attention to progress during the current term to address challenges and achieve targets in product development, clinical development, regulatory affairs, and business development.
|
| <Reference: regarding corporate governance> |
 ◎ Corporate governance reports
Updated on July 5, 2017
<Reasons for Non-Compliance with the Principles of the Corporate Governance Code (Excerpts)>
It is mentioned that "Our company follows all of the basic principles of the Corporate Governance Code." ◎ Corporate governance reports
Updated on July 5, 2017
<Reasons for Non-Compliance with the Principles of the Corporate Governance Code (Excerpts)>
It is mentioned that "Our company follows all of the basic principles of the Corporate Governance Code."
Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation for investment. The information and opinions contained within this report are made by our company based on data made publicly available, and the information within this report comes from sources that we judge to be reliable. However, we cannot wholly guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2018 Investment Bridge Co., Ltd. All Rights Reserved. |




