| CellSeed (7776) |
|
||||||||
Company |
CellSeed Inc. |
||
Code No. |
7776 |
||
Exchange |
JASDAQ |
||
Industry |
Regenerative Medicine |
||
President |
Yukio Hasegawa |
||
HQ Address |
R-Bldg., Shinjuku 1F, 33-8, Wakamatsu-cho, Shinjuku-ku, Tokyo, 162-0056, Japan |
||
Year-end |
December |
||
URL |
|||
* Stock price as of closing on 2011/5/18. Number of shares at the end of the most recent quarter excluding treasury shares.
|
||||||||||||||||||||||||
|
|
Mail: CellSeed@cyber-ir.co.jp |
|
| Key Points |
 |
| Company Overview |
|
Regenerative Medicine Support Business
CellSeed develops and manufactures the temperature responsive cell cultureware and various applications of this product (Some of the manufacturing processes that require large capital investments are outsourced.) that are the fundamental tool in cell sheet regenerative medicine. These products are provided to universities, research institutions and other institutions around the world. This business is of high strategic importance in developing partners in the cell sheet regenerative medicine business, in addition to its main purpose of generating earnings.
Cell Sheet Regenerative Medicine Business
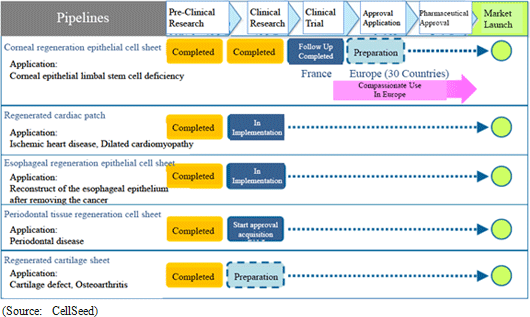
Cell sheet regenerative medical products (Cell sheets) and various applications of these products are sold in this business. Currently CellSeed is conducting joint research and development of five different regenerative medical product pipelines with Tokyo Women' s Medical University, Osaka University, and Tokai University.
<Product Pipelines Conditions in First Quarter of Fiscal Year December 2011>
 |
| First Quarter Fiscal Year December 2011 Earnings Results |
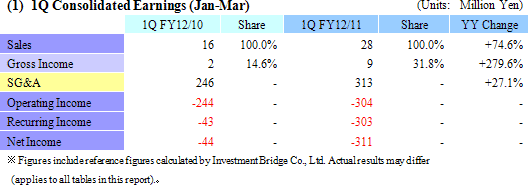 Sales Rise by 74.6% YY, Recurring Loss of ¥300 Million (Compared with ¥43 Million Loss in Previous Term)
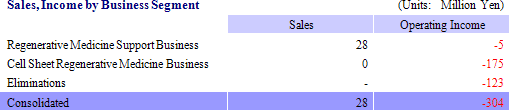
While there were no sales in the cell sheet regenerative medicine business because it is still in the pre-commercial launch phase, sales of new products launched last summer including the cell tight junction real monitoring system "cellZscope" helped to boost sales of the regenerative medicine support business to ¥28 million from ¥16 million in the same term of the previous year. With regards to profits, increases in SG&A costs including primarily research and development (¥136 to ¥175 million) contributed to the higher levels of operating losses, but these factors remained within CellSeed' s expectations. Non-operating income deteriorated due to a decline in subsidies from ¥226 to ¥0.8 million, and an extraordinary loss of ¥6 million resulted from the implementation of asset retirement obligation accounting standards.
  (2) Topics During the First Quarter
[1] Joint Research Agreement with the LA Biomedical Research Institute
In February CellSeed entered into an agreement (Covering a two year period) to conduct joint research in regenerative medical applications using cell sheet regenerative engineering that are a fundamental part of regenerative medical technologies with Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (LA Biomed), one of the largest non-profit medical institutions in the United States.LA Biomed was established in 1952 in Torrance, California as a non-profit medical institution and employs over 150 senior researchers who conduct research projects designed to develop new treatments for patients suffering with cancer, hereditary diseases, infectious diseases and other diseases (Research on over 1,000 themes is being conducted.). In addition to close technological collaboration with the David Geffen School of Medicine at UCLA, LA Biomed also trains young technicians, provides immunization programs, provides instruction on infant nutrition, and maintains a goal of contributing to the medical community (LA Biomed has established a position as the leading institution in the field of medicine and medical care in the south Los Angeles area.). A team led by Yutaka Niihara, PhD. (Professor at UCLA Medical School) is undertaking joint research, and CellSeed hopes to use this joint research to promote the commercial launch of its corneal regeneration epithelial cell sheet, which is currently undergoing preparation for commercial launch in Europe, in the United States at an early stage as well. CellSeed also hopes to promote other various applications of the cell sheet regenerative medicine business in the United States. [2] Joint Research and Development for Self Cultured Corneal Epithelial Layer with Japan Tissue Engineering Co., Ltd. (J-TEC)
In January CellSeed formed an "individual agreement for joint research and development" (Covering a three year period) for joint research of self cultured corneal epithelial layer with J-TEC. A "basic agreement for joint research and development" had been formed between both companies in October 2010, and CellSeed had been conducting joint research with J-TEC based on its technology and knowhow in the cell sheet regenerative medicine field and related products. During the course of this research, CellSeed was able to confirm promising basic data on the efficacy of the use J-TEC' s self cultured corneal epithelial layer materials in CellSeed' s own temperature responsive cell cultureware. Consequently, CellSeed decided to form a separate agreement with J-TEC to apply its own cell sheet regenerative medicine to J-TEC' s self cultured corneal epithelial layer materials technology.Furthermore J-TEC was able to successfully launch the first regenerative medicine product within Japan as a commercial business, and is the only company in Japan with the capacity to produce regenerative medical products. In accordance with the "individual agreement for joint research and development," J-TEC is promoting research of products which have been revised using self cultured corneal epithelial layer, and endeavoring to quickly submit full performance confirmation approval application for these products. The formation of an "individual agreement for joint research and development" marks the first concrete step for CellSeed (The joint research and development conducted with J-TEC, which boasts of superior track record and facilities, is considered to be an important strategy.) in its cell sheet regenerative medicine business in Japan. [3] Regenerated Cardiac Patch Basic Patent Approval
Basic patents for regenerated cardiac patch using cell sheet regenerative engineering were granted to CellSeed in Europe in January and in Japan in March.Amongst the various product pipelines that use cell sheet regenerative engineering that are currently being pursued by CellSeed, regenerated cardiac patch is the product pipeline with the largest market potential and these patents are a reflection of the revolutionary technology behind the basic concept of this product (Cell sheets are cultured from heart tissue cells of the patient and then transplanted to the patient' s heart to help fortify its function.). With regards to patients with severe ischemic heart disease and dilated cardiomyopathy, there is currently no effective treatment for these ailments (Treatments are limited to heart transplant from donors. Therefore most of the treatments are designed as "temporary" measures to maintain the health of the patient while they await a donor heart.). At the same time, CellSeed' s regenerated cardiac patch is designed to be a fundamental solution that uses cell sheet regenerative medicine products based on a regenerated cardiac patch that is cultured from the patients' own cells and then grafted back to their heart to restore its function. Currently CellSeed is promoting joint research to realize practical use of the regenerated cardiac patch and the approval of their patents for regenerated cardiac patch is an indication of the revolutionary nature of this technology. The approved patent is effective in 10 countries in Europe including the United Kingdom, Germany, France, Italy, Spain, Holland, Belgium, Sweden, Finland and Greece. Dilated cardiomyopathy is a disease that causes the left chambers of the heart to dilate and leads to malfunction of this portion of the heart. Normally cardiac arrest symptoms accompanying congestion are apparent, and in some cases fatigue resulting low output state can be apparent. Ischemic heart disease is the condition where the heart is damaged due to restriction of blood flow due to blockage or constriction of the coronary artery and includes cardiac angina and cardiac infarction. In addition, Professor Teruo Okano, who is also an outside director of CellSeed and the leading academic in the field of cell sheet regenerative medical technologies, was interviewed in a television program called "Tradition of a Professional" (Professional Shigoto No Ryugi) that aired on Nippon Hoso Kyokai (Japan Broadcasting Corporation, the Government run television station) in January. |
| Fiscal Year December 2011 Earnings Estimates |
|
(1) Basic Agreement for Joint Research and Basic Agreement for Joint Research and Development, and Commercialization of Corneal Epithelial Cell Sheet Technologies Signed with Emmaus Medical Inc.
On April 8 CellSeed signed agreements with Emmaus Medical, Inc. (Torrance, California, U.S.) for "basic agreement for joint research and development" and "agreement for joint research and development and commercialization of corneal epithelial cell sheet technologies".
[1] About Emmaus Medical, Inc.
Emmaus Medical was established in California in 2003 by Yutaka Niihara, PhD. (Professor at UCLA Medical School) and others with the mission of developing applications using L-glutamine (A type of amino acid found commonly in muscle tissue that was discovered by Yutaka Niihara.) that is effective in the treatment of difficult diseases (drepanocyte disease, short-bowel syndrome, and others). Applications of L-glutamine effective in the treatment of SBS have already launched into the market, and clinical trials of applications in the treatment of drepanocyte disease are currently being conducted (Currently in phase 3 clinical trial).Emmaus Medical is gathering the attention of the medical community not only in the United States, but in Japan and other parts of the world as well. Henry A. McKinnell Jr., PhD., who used to be the CEO of Pfizer Inc., is also an external director. Emmaus Medical was capitalized at US$13.8 million and held 2.7% of CellSeed' s outstanding shares as of end December 2010. [2] Ongoing Cooperation to Leverage the Strengths of Both Companies - CellSeed and Emmaus -Maintaining the Spirit, Mission and Vision of Venture Companies
CellSeed and Emmaus Medical are companies created by Japanese with revolutionary new medical technological foundations and they share the goal of contributing to society by promoting the diffusion of leading edge medical treatments and products throughout the world. Moreover the two companies share many characteristics including the fact that they are both medical venture companies managed by Japanese. In addition, while CellSeed is a Japanese company with regenerative medical platform technologies endeavoring to launch its business in Europe, Emmaus Medical is a United States company with a strong network and research capabilities with products already launched in the market. Also both companies are cooperating to supplement each other' s business operations and research and development activities on a global basis with a view to expanding into Africa and other developing economy markets.
[3] Overview of Contracts and Impact Upon Earnings
The "basic agreement for joint research and development" is an agreement to conduct joint research and development in the United States for cell sheet regenerative medicine products that employ cell sheet engineering. The concrete details of the joint research and development are explained separately as defined in the contract. Because CellSeed will provide knowledge and knowhow to Emmaus Medical going forward in accordance with the basic contract and individual contract, CellSeed will receive US$8.5 million (¥720 million using recent exchange rate.) as a lump sum payment (For the term of the contract effective as of the signing of the contract and ending at the end of the contract.). Moreover for the individual contract formed under the "basic agreement for joint research and development" contract, CellSeed will receive a US$1.5 million (¥127 million using recent exchange rate.) lump sum payment (As the compensation for the disclosure of research achievement of corneal regeneration epithelial cell sheets.).The US$8.5 million lump sum payment for the "basic agreement for joint research and development" has not been included in its current term earnings estimates, and CellSeed is currently consulting with accountants to determine whether it should be included in fiscal year December 2011 earnings. At the same time CellSeed expects to include the lump sum payment of US$1.5 million from its individual agreement in its second quarter earnings, and has therefore revised its earnings estimates upwards to reflect this development. 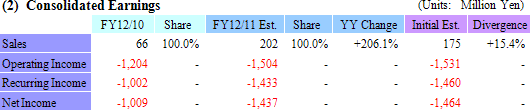 Sales of ¥200 million and Recurring Loss of ¥1.43 billion Expected
Sales are expected to rise by 206.1% year-over-year to ¥202 million, with the regenerative medicine support business and cell sheet regenerative medicine business contributing ¥68 and ¥134 million in sales respectively. While the lump sum payment from the individual agreement for the joint research and development of corneal regeneration epithelial cell sheet product in the United States was included in earnings estimates, the payment exceeded expectations by ¥27 million and the full year sales estimates for the cell sheet regenerative medicine business were revised up from the initial estimate of ¥107 million. At the same time while sales of new products trended above initial estimates in the regenerative medicine support business, uncertainties caused by the Great Eastern Japan Earthquake and Tsunami disaster and subsequent potential disruption in electric power, distribution functions and other issues led CellSeed to maintain its outstanding estimates of ¥68 million. With regards to profits, the upward revision in sales of cell sheet regenerative medicine business allowed this division' s income to be revised up by ¥27 million as well, while estimates for income in the regenerative medicine support business were left unchanged.
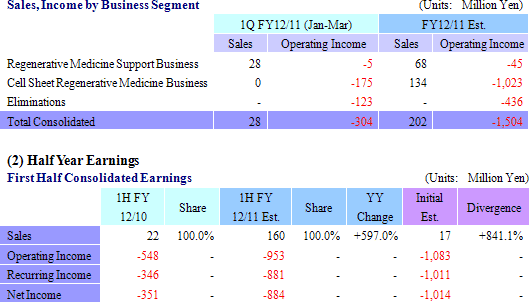 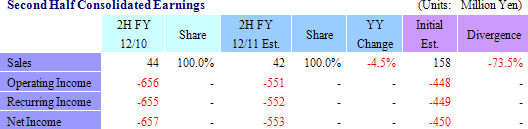 (2) Topics Expected from the Second Quarter Forward
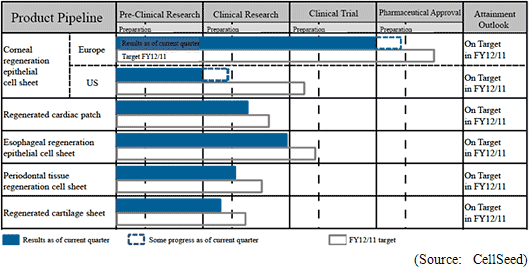
With regards to the corneal regeneration epithelial cell sheet related business, the results of the clinical trials conducted in France are currently being reviewed and an application for commercial launch is expected to be submitted to the European Medicines Agency based on these results. In addition, provision of products on a "compassionate use basis" is expected to begin, along with the payment of a lump sum fee expected to be received for joint development with Emmaus Medical in the United States.With regards to the cell sheet regenerative medicine pipeline, lump sum payments for the basic agreement for joint research and development with Emmaus is anticipated, and preparation for the start of clinical research for esophageal regeneration epithelial cell sheet and regenerated cartilage sheet is expected to be completed.  |
| Conclusions |
|
In addition to returns to investors, the success of CellSeed' s businesses will also contribute to society by improving the "quality of life" of patients suffering from difficult diseases around the world. Furthermore CellSeed will continue to pursue the development of medical products and technologies that are high in value addition as part of their efforts to contribute to society. Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2011, All Rights Reserved by Investment Bridge Co., Ltd. |



