| CellSeed Inc. (7776) |
|
||||||||
Company |
CellSeed Inc. |
||
Code No. |
7776 |
||
Exchange |
JASDAQ |
||
Industry |
Precision Instrument (Manufacturing) |
||
President |
Setsuko Hashimoto, Ph.D. |
||
HQ Address |
Telecom Center Building, Aomi 2-5-10, Koto-ku, Tokyo |
||
Year-end |
December |
||
URL |
|||
* Stock price as of closing on February 24, 2017. Number of shares at the end of the most recent quarter excluding treasury shares.
|
||||||||||||||||||||||||
|
|
* Estimates are created by the Company. Since the FY12/16, the definition of net income has been changed to profit attributable to parent company shareholders (Abbreviated as parent net income).
This Bridge Report presents analysis of the fiscal year December 2016 earnings results and a review of the Mid-term Business Plan (From FY12/17 to FY12/19) for CellSeed Inc. |
|
| Key Points |
 |
| Company Overview |
|
<Regenerative Medicine and CellSeed's Business Strategies>
Regenerative medicine is a form of medical treatments that can regenerate organs that have reduced functions, been damaged, or lost. Stem cells are cells that can be differentiated into various cells to be used as key components within regenerative medicine technology applications. Currently, there are three types of stem cells including "ES cells" created from fertilized eggs, "iPS cells" induced pluripotent cells, and "organ stem cells" from various living organs. And while "ES cells" created from fertilized eggs have the ability to be differentiated into all cell types, there are ethical issues regarding the use of fertilized eggs and they have yet to become commercialized due to this issue.
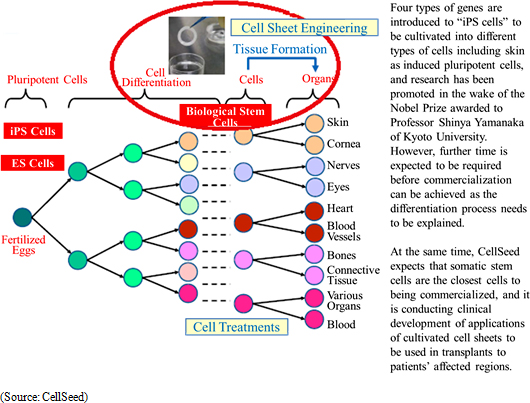 <Regenerative Medicine Fundamental Technologies - "Cell Sheet Engineering">
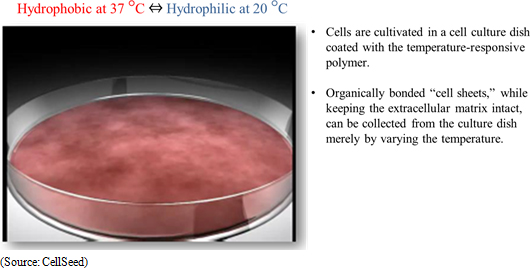
"Cell sheet engineering" is the world's first platform technology for regenerative medicine developed in Japan. Regenerative medicine uses cultured cells, but conventional technologies led to damage of cells during the culturing process. Compared with these conventional technologies, CellSeed's innovative Cell sheet engineering allows cells to be cultured without being damaged. The main point behind this technology is the use of cell cultureware that uses temperature-responsive polymer on the surface of cell cultureware. These temperature-responsive polymers exhibit hydrophobic properties at 37 degrees Celsius, which is the same as body temperature, and hydrophilic properties at 20 degrees Celsius which allow the attached cells sloughing off naturally from the surface and enable collection of cells at these temperatures without being damaged.
 <Development of Treatments Using Cell Sheet Engineering>
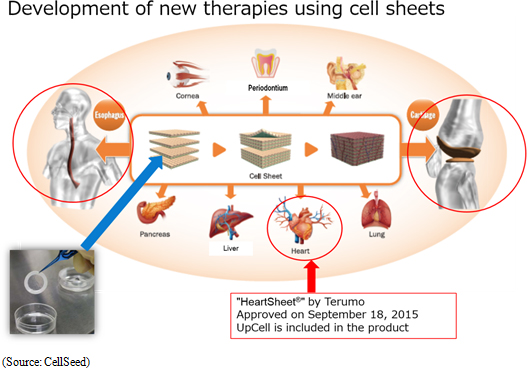 Moreover, Terumo Corporation's "Heart Sheet" (Regenerated heart tissue patch) uses CellSeed's "UpCell" temperature-responsive cell cultureware and has received approval on a limited time basis. CellSeed signed a "fundamental agreement for provision of cell cultureware" with Terumo at the end of March 2016, and as part of this agreement will provide Terumo with "UpCell" temperature-responsive cell cultureware. Approval on a limited time basis is part of an "expedited approval system" defined by the Pharmaceutical and Medical Devices Act that enables evaluation of efficacy and safety in a short period of time. At the same time, further validation of efficacy and safety of treatments is conducted after market launch with a second round of approval being required within five years after the market launch under this limited time basis approval system. |
| Fiscal Year December 2016 Earnings |
 Cell Processing Facility Acquisition Cost Written Off as Onetime Expense
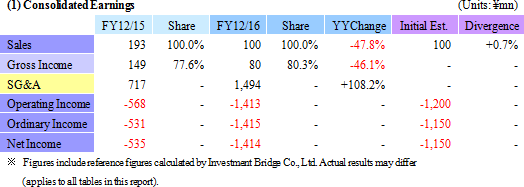
Sales declined by 47.8% year-on-year to ¥100 million, with ¥50 million in sales being booked in both the Cell sheet regenerative medicine and Intelligent cell cultureware business respectively. With regard to profit, the lower sales led to a decline in gross income, and increases in research and development expenses from ¥302 to ¥1,065 million caused sales general and administrative expenses to rise from ¥568 to ¥1,494 million. Consequently, the operating loss expanded by 2.5 times year-on-year to ¥1.412 billion. The main reason behind this increase in research and development expenses was the ¥524 million acquisition of cell Cell Processing Facility, which was written off as a onetime expense.
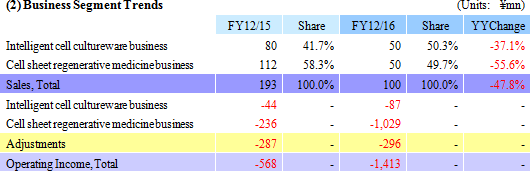 Intelligent cell cultureware business
While higher sales had been anticipated, only ¥50 million in sales of temperature-responsive cell cultureware were booked due to the termination of some research projects and reductions in budgets by existing customers (Universities, research institutions).
Cell Sheet Regenerative Medicine Business
Negotiations with MetaTech(AP) Inc., which is a publicly traded company listed on the Over The Counter Market in Taiwan, have been started in December 2016 for the launch of cell sheet regenerative medicine business in Taiwan, with a derivation study deposit fee of ¥50 million received (¥112 million was booked in the previous term as a part of the prepayment accounts received in March 2012 as a result of the agreement with Emmaus Medical Inc. in the United States for joint development of epithelial cell sheet for corneal regeneration and fundamental contract for joint research and development).With regard to research and development, clinical trial notification was submitted in April 2016 and the clinical trials for "esophageal regeneration epithelial cell sheet" were started in August 2016 with the National Cancer Center and the Tokyo Women's Medical University. In addition, CellSeed signed a "fundamental agreement for provision of cell cultureware" in March 2016 to provide Terumo with CellSeed's "UpCell" for use in Terumo Corporation's "Heart Sheet" (Regenerated heart tissue patch). Also, a patent licensing agreement for corneal regeneration epithelial cell sheet was formed with Emmaus Medical Inc. of the United States in June 2016. 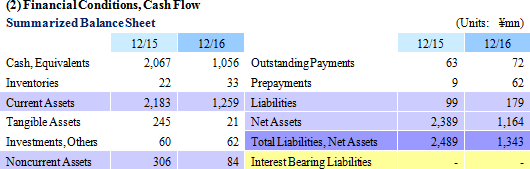 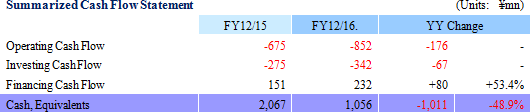 |
| Strategies to Achieve Targets of the Mid-term Business Plan (Fiscal Years December 2017 to 2019) |
|
<Mid-term Business Plan Overview, Numerical Targets>
Mid-term Business Plan Overview 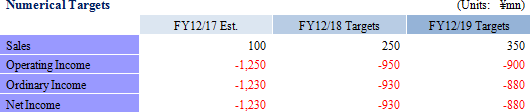 <Strategies to Achieve Targets of the Mid-term Business Plan>
According to CellSeed, approximately 22,000 patients within Japan are diagnosed with esophageal cancer every year (90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma), with approximately 11,500 patients dying every year. In addition, the rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients, with five year survival rates for males and females said to be 36% and 44% respectively. According to the 2008 insurance listings, the number of endoscopic resection surgeries (ESD) is on the rise and has brought on cases of esophageal stricture as a side effect. CellSeed maintains a mission of contributing to the curing of esophageal cancer by introducing its esophageal regeneration epithelial cell sheet in endoscopic resection surgeries implemented in early detection cases. (1) "Esophageal Regeneration Epithelial Cell Sheet" and "Regenerated Cartilage Sheet" Sales Approval Acquisition "Esophageal Regeneration Epithelial Cell Sheet" "Esophageal regeneration epithelial cell sheet" for esophageal cancer regeneration treatments (Esophageal wound treatment, esophageal stricture prevention) has been developed at the Institute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical University. Cells taken from patient's mucosal membrane are cultivated in temperature-responsive cultureware for approximately two weeks and turned into cell sheets. Cell sheets are cultured to be used to replace parts of the esophagus that are infected by cancer and removed during surgery. Clinical research was conducted from 2008 to 2014 with 10 cases studied at the Tokyo Women's Medical University, 10 at Nagasaki University (Remote validation of cells acquired at Nagasaki University and cultured at Tokyo Women's Medical University, and then grafted to patients at Nagasaki University), and another 10 at Karolinska University Hospital (Sweden) for a total of 30 cases. CellSeed has established a basic agreement for development of applications with Tokyo Women's Medical University and the successful results of joint research with this institution are being leveraged in new application development. 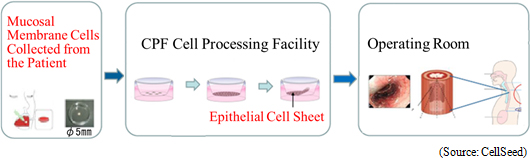 Clinical Trials Start from August 2016
Clinical trials conducted at the National Cancer Center Japan (2 facilities including the Chuo and East Hospitals) and Tokyo Women's Medical University started in August 2016 after notification of launch of clinical trials was submitted in April 2016 (9 Phase III clinical trials are planned to be implemented). In addition, clinical trials are expected to be conducted in Sweden by CellSeed Sweden AB, which was established in May 2015, and prior consultations with the Swedish Medical Products Agency have begun from November 30, 2015. Also, prior consultations with the European Medicines Agency for preparation of clinical trials on a European-wide basis have also begun.
Simultaneous Development of Cell Sheet Transplant Devices
Because of the need for highly advanced technology for use in esophageal regeneration epithelial cell sheet transplants, CellSeed has developed a cell sheet transplant device that reduces surgery workloads. CellSeed has acquired approval for esophageal regeneration epithelial cell sheet in combination with this device (Clinical trials are being conducted jointly for both cell sheets and this device), but approval for sale and use in Europe still needs to be acquired. Therefore, efforts are now being conducted to acquire the CE mark (Approval for medical equipment in Europe).
 "Regenerated Cartilage Sheet"
Regenerated cartilage sheet is the result of research conducted jointly by CellSeed and Tokai Medical University School of Orthopedics Professor Masato Sato, and is a treatment designed to be used on patients with damaged and deformed cartilage due to sports related injuries and osteoarthritis due to aging (Estimated number of patients is 12.00 million). While there are no effective treatments for either ailment currently available, joint research being conducted with Professor Sato of Tokai Medical University with a target of fundamentally regenerating cartilage surface has been implemented in eight cases of clinical research (Knee cartilage is known as "glass cartilage" and differs from cartilage of the ear and nose in its superior cushioning and wear functions. A unique characteristic of CellSeed is its ability to regenerate this "glass cartilage" in the form of regenerated cartilage sheets.). Strategic consultations with the Pharmaceuticals and Medical Devices Agency (PMDA) responsible for granting sales approval have begun. But further consultations will be conducted along with efforts to aggregate safety data and establish clinical protocols. Moreover, DNA Chip Research Inc. and Tokai University will participate in the "Self-Cartilage Cell Sheet Integrated Evaluation Method" Project of the Japan Agency for Medical Research and Development (AMED). 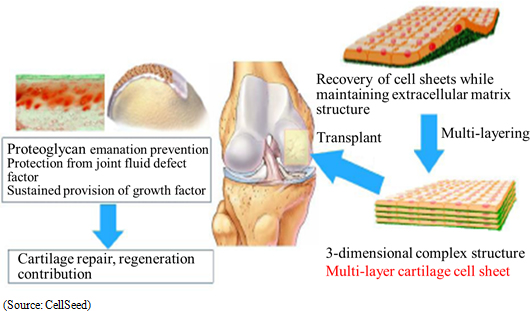 Joint Research with Tokai University
CellSeed began providing temperature-responsive cell cultureware and joint research with Tokai University in 2004 and 2006 respectively. From 2010 onwards, the Company has supported clinical research conducted by and dispatched researchers to Tokai University, as part of the joint research structure. With regard to clinical research, research on transplant of cell sheets made from patients' own cells received the approval of the 65th Scientific Technology Committee of the Ministry of Health, Labor and Welfare Medical Care Deliberation Committee in August 2011. In October 2011, the Minister of Health, Labor and Welfare also issued a written opinion (MHLW Legal Document 1003 Number 3), and the first case of clinical research was begun in November 2011. Furthermore, evaluations of 8 cases were completed in November 2015, with favorable post surgery results in these cases having been observed in transplant patients three years after surgery).With regards to allogeneic cell sheet transplants, the first case of clinical research was started on February 15, 2017 (Transplant surgery implemented). In this research, cartilage tissue was taken from the patient polydactyly and cultivated over a two to three week period to become a cell sheet used for the transplant (Cartilage tissue from the finger surgically removed from a child with a congenital condition born with six fingers was used by consent). Over the next three years, transplant surgery is expected to be performed on 10 patients. Moreover, CellSeed and Tokai University have established a patent jointly within Japan and their overseas patent application is currently being considered. Furthermore, they expect to expand the patent network even further. (2) Deliberations for Business Alliance in Taiwan
CellSeed has started deliberations in December 2016 with MetaTech (AP) Inc. for the launch of its cell sheet regenerative medicine business through an alliance in Taiwan. Also, MetaTech has paid ¥50 million in mobilization fees for the consideration of the business start with CellSeed.MetaTech is a wholesaler and retailer of electronic materials, medical and beauty products, and medical equipment, and is a publicly traded company listed on the Over The Counter Market in Taiwan providing biotechnology and other services there. The current deliberations have been initiated by MetaTech based upon its desire to become the sole company conducting business deployment of the cell sheet (esophageal regeneration epithelial cell sheet and regenerated cartilage sheet) in Taiwan. Furthermore, this potential business alliance is in keeping with CellSeed's Mid-term Business Plan for the overseas deployment of its cell sheet regenerative medicine business. As the first step, MetaTech is expected to sign a contract with CellSeed for it to provide a package of information necessary to create a business plan for the cell sheet regenerative medicine business in Taiwan. This contract is expected to be concluded in March 2017. MetaTach was established on September 17, 1998, and boasted of sales, ordinary income, total assets, net assets, and capital of Taiwan $2.153 billion, $6 million, $1.033 billion, $524 million and $4 million respectively (At an exchange rate of ¥3.65 per Taiwan Dollar: ¥7.858, ¥0.022, ¥3.770, ¥1.913 and ¥0.160 billion respectively). (3) Cell Processing Facility (CPF) Completed
The cell sheet cultivating facility known as the Cell Processing Facility (CPF) has been completed on the sixth floor of the Telecom Center Building, which is also the location of CellSeed's headquarters in Aomi, Koto Ward, Tokyo. This Cell Processing Facility boasts of 763 square meters in floor space, and has automated monitoring systems to monitor and control cleanliness, room pressure, temperature and humidity, devices (cultureware, cold storage, etc.), and a surveillance camera system throughout the entire facility. In addition, this facility is ideally located only 20 minutes away from Haneda International Airport. Also, an application for certification as an officially recognized cell sheet regenerative medicine manufacturing facility that complies with the Act of Ensuring Safety in Regenerative Medicine has been submitted.
 (4) Strengthening the Intelligent cell cultureware business
Efforts are being made to fortify the marketing strategy and to expand opportunities to capture earnings by promoting development of new products and new applications for existing products. With regard to efforts to expand opportunities to capture earnings by promoting development of new products and new applications of existing products, development of clinical response application products and new products based upon existing research use material products are being conducted. One example of an applied use for clinical products is the provision of temperature-responsive cell cultureware specialized for use in Terumo's "Heart Sheet" product. With regard to efforts to fortify CellSeed's marketing strategy, efforts are being made to strengthen sales channel within Japan and in overseas markets, strengthen customer support capabilities, and to participate in exhibitions and host seminars. With regard to domestic sales, CellSeed currently relies upon two distributors, but the number of companies making inquiries to become distributors is on the rise. In overseas markets, the Company will identify distributors in each respective country. In addition to fortifying customer support and support responses for new research, CellSeed will develop new products that respond to the customer needs including easier to use products. Furthermore, the Company will increase the number of exhibitions in which it participates and seminars which it hosts.
(5) Funding Conditions
CellSeed will conduct acquisition and elimination of stock options for new shares (13th) on March 6, 2017 for which exercise of options to be converted to shares became difficult due to declines in the share price, along with the issuance of a third party placement of stock options for new shares (16th).
Acquisition, Elimination of Unexercised Stock Options
CellSeed issued its 13th round of third party placement of stock options for new shares on August 31, 2015 to Milestone Capital Management. The Company was able to raise ¥373 million through the exercise of 530 options, but the sustained trading of the share price below the exercise price has made the outlook for exercise of the options difficult. Consequently, CellSeed has acquired the outstanding unexercised stock options on March 6, 2017 and eliminated them.
New Third Party Placement of Stock Options
On February 17, 2017, CellSeed concluded an agreement for third party placement of stock options for new shares with Evolution Biotech Fund. The funds derived from this agreement will be used as capital for the Intelligent cell cultureware business, general working capital, operating capital for the Cell Processing Facility and for investments to develop the business structure in Taiwan. Details of the stock options for new shares are provided below.
First Half Commitmentre:Committed to exercise 50% or more of the number of optioned shares that have been issued within 146 price determination days |
| Future Highlights |
|
|
| <Reference: CellSeed's corporate governance> |
 ◎Corporate Governance Report
Latest Update: April 1, 2016
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people's health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more. Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2017 Investment Bridge Co., Ltd. All Rights Reserved. |



