| CellSeed Inc. (7776) |
|
||||||||
Company |
CellSeed Inc. |
||
Code No. |
7776 |
||
Exchange |
JASDAQ |
||
Industry |
Precision Instrument (Manufacturing) |
||
President |
Setsuko Hashimoto, Ph.D. |
||
HQ Address |
Telecom Center Building, Aomi 2-5-10, Koto-ku, Tokyo |
||
Year-end |
December |
||
URL |
|||
* Stock price as of closing on August 24, 2017. Number of shares at the end of the most recent quarter excluding treasury shares.
|
||||||||||||||||||||||||
|
|
* Estimates are created by the Company. Since the FY12/16, the definition of net income has been changed to profit attributable to parent company shareholders (Abbreviated as parent net income).
This Bridge Report presents analysis of the first half of fiscal year 2017 earnings results and an overview of the full year earnings estimates for CellSeed Inc. |
|
| Key Points |
 |
| Company Overview |
|
The cell sheet cultivating facility known as the Cell Processing Facility (CPF) has been completed in August 2016 on the sixth floor of the Telecom Center Building, which is also the headquarters of CellSeed's in Aomi, Koto Ward, Tokyo. This Cell Processing Facility boasts of 763 square meters of floor space, and has automated systems to monitor to control cleanliness, room pressure, temperature and humidity, and a surveillance camera system throughout the entire facility. In addition, this facility is ideally located only 20 minutes away from Haneda International Airport. In March 2017, "Specialized Cell Processing Manufacturing Certification" approval in accordance with Article 35, paragraph 1 of the Act of Ensuring Safety in Regenerative Medicine was granted by the Ministry of Health, Labour and Welfare. Consequently, CellSeed is considering consigned processing business for cell sheets needed for clinical research, clinical trials, medical care not covered by health insurance, and other medical processes. <Regenerative Medicine and CellSeed's Business Strategies>
Regenerative medicine is a form of medical treatment that can regenerate organs that have reduced, damaged, or lost functions. Stem cells are cells that can be differentiated into various cells to be used as key components within regenerative medicine technology applications. Currently, there are three types of stem cells including "ES cells" created from fertilized eggs, "iPS cells" induced pluripotent cells, and "organ stem cells" from various living organs. And while "ES cells" created from fertilized eggs have the ability to be differentiated into all cell types, there are ethical issues regarding the use of fertilized eggs and they have yet to become commercialized due to this issue. Four types of genes are introduced to "iPS cells" to be cultivated into different types of cells including skin as induced pluripotent cells, and research has been promoted in the wake of the Nobel Prize awarded to Professor Shinya Yamanaka of Kyoto University. However, further time is expected to be required before commercialization can be achieved as the differentiation process needs to be explained.At the same time, CellSeed views somatic stem cells as the closest cells to being commercialized, and it is conducting clinical development of applications of cultivated cell sheets to be used in transplants to patients' affected regions (Esophageal and knee cartilage regenerative medical products). Specifically, approval applications for sale of "epithelial cell sheets for esophageal regeneration" treatments and for start of clinical trials for "regenerated cartilage sheet" are expected to be submitted in 2017 with sales approval application expected to be submitted in 2018. Clinical data for regenerative medical products using "cell sheet engineering" including not only esophagus and knee cartilage, but also cornea, teeth, ear, cartilage, lung, heart, liver and pancreas applications have been gathered. Moreover, Terumo Corporation's "Heart Sheet" (Regenerated heart tissue patch) uses CellSeed's "UpCell" temperature-responsive cell cultureware and has received approval on a limited time basis in September 2015. CellSeed provides "UpCell" temperature-responsive cell cultureware for "Heart Sheet". |
| First Half of Fiscal Year 2017 Earnings |
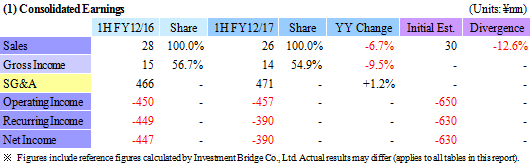 Efficient Operations of Cell Processing Facility (CPF), Delays in Research and Development Allow for Smaller than Anticipated Loss
CellSeed was able to book ¥26 million in sales in its regenerative medicine supporting business. While these sales fell shy of estimates, this shortfall is attributed to delays in filling product orders booked during the first half into the second half. At the same time, better than expected operating efficiency of its Cell Processing Facility (CPF) and delays in booking of research and development expenses into the second half allowed for a smaller than expected operating loss to be recorded. Research and development expenses rose by 7.4% year-on-year to ¥251 million.Moreover, CellSeed was selected as the main institution by the National Research and Development Agency and the Japan Agency for Medical Research and Development for the "Evaluation Platform Technologies for Regenerative Medicine Commercialization Project" in fiscal year 2017, along with Tokai University and DNA Chip Research Inc. for work on "Development of Efficacy and Quality Assessment Method for Allogeneic Cartilage Cell Sheet" (In the future, partial subsidies are expected to be received for research and development expenses). In addition, CellSeed also participated in the "2017 Bio International Exhibition" held in San Diego, California from June 17 to 20, 2017. (2) Fiscal Year 2017 Earnings Estimates
CellSeed maintained its outstanding earnings estimates for the full year and expect sales of ¥100 million and operating, recurring and net losses of ¥1.250, ¥1.230 and ¥1.414 billion to be achieved respectively (Compared with sales, and operating, recurring and net losses of ¥0.100,¥1.413, ¥1.230, and ¥1.415 billion in the previous fiscal year December 2016).
|
| Progress in the Mid-term Business Plan (From FY12/17 to FY12/19) |
|
<Mid-term Business Plan Overview>
Progress in the 6 Pillars of the Business Plan  <Cell Sheet Regenerative Medicine Pipeline>
Esophageal cancer regeneration treatments (Esophageal wound treatment, stricture prevention) have been developed at the Institute of Advanced BioMedical Engineering and Science, Tokyo Women's Medical University. Cells taken from patient's mucosal membrane are cultivated in temperature-responsive cell cultureware for two weeks and turned into cell sheets. Cell sheets are cultured to be used to replace parts of the esophagus that are infected by cancer and removed during surgery.(1) "Epithelial Cell Sheets for Esophageal Regeneration" According to CellSeed, 22,000 patients within Japan are diagnosed with esophageal cancer every year (90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma), with 11,500 patients dying every year. In addition, the rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients, with five year survival rates for males and females said to be 36% and 44% respectively. The endoscopic resection surgeries (ESD) was posted in the drug price list from 2008 and is on the rise (About 20% of patients diagnosed with esophageal cancer undergo surgery) and cases of esophageal stricture after surgery has been noted as a side effect. 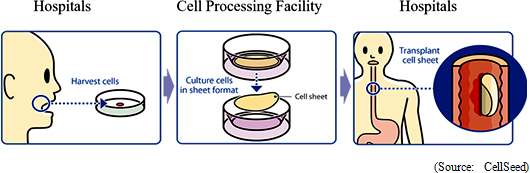 Sakigake Designation Scheme System Products Specified
CellSeed's regenerative medicine technology was selected for the scope of the "Sakigake Designation Scheme System" by the Ministry of Health, Labour and Welfare. This system is designed to allow patients within Japan to access the world's cutting edge treatments at the earliest stage. The scope of the this system includes treatments that meet the four criteria of 1) epoch making new mechanism of action, 2) applications for patients with severe diseases, 3) high levels of efficacy, and 4) potential for early development and approval within Japan ahead of the rest of the world. Treatments selected by this system receive preferential treatment in consultations and deliberations for pharmaceutical approval, and instructions on manufacturing facilitation post-approval for the smooth introduction into the medical market (Merits of this system include preferential consultations, preliminary evaluation, prioritized reviews, review partner system).
(2) Regenerated Cartilage Sheet
Regenerated cartilage sheet is the result of research conducted jointly by CellSeed and Tokai Medical University School of Orthopedics Professor Masato Sato, and is a treatment designed to be used on patients with damaged and deformed cartilage due to sports related injuries and osteoarthritis due to aging. While there are currently available no effective treatments for either ailment, research is being conducted jointly with Professor Sato with a target of fundamentally regenerating cartilage surface. Knee cartilage is known as "glass cartilage" and differs from cartilage of the ear and nose in its superior cushioning and wear functions. However, regeneration of "glass cartilage" has been confirmed in jointly conducted clinical research using "regenerated cartilage sheet".
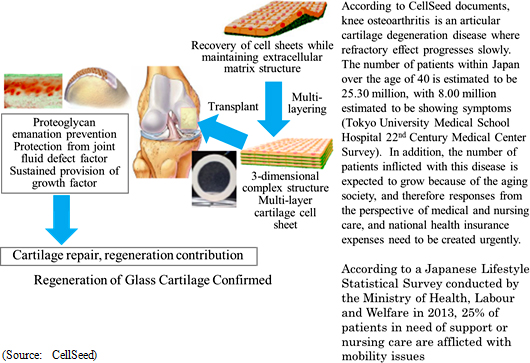 Joint Research with Tokai University
Clinical research on transplant of cell sheets made from patients' own cells received the approval of the 65th Scientific Technology Committee of the Minister of Health, Labour and Welfare Medical Care Deliberation Committee in August 2011, and the Minister of Health, Labour and Welfare also issued a written opinion (MHLW Legal Document 1003 Number 3) in October 2011. Furthermore, two-year evaluations of 8 cases were completed in November 2015 (Favorable post surgery results in these cases having been observed in transplant patients three years after surgery). Strategic consultations with the Pharmaceuticals and Medical Devices Agency (PMDA) responsible for granting sales approval have begun. But further consultations will be conducted along with efforts to aggregate safety data and establish clinical protocols.With regards to allogeneic cell sheet transplants, the first case of clinical research was started on February 15, 2017 (Transplant surgery implemented). In this clinical research, cartilage tissue was taken from the patient polydactyly and cultivated over a two to three week period to become a cell sheet used for the transplant (Cartilage tissue from the finger surgically removed from a child with a congenital condition born with six fingers was used by consent). Over the next three years, transplant surgery is expected to be performed on 10 patients. Cultivation of cells at the Cell Processing Facility (CPF) is under discussion. 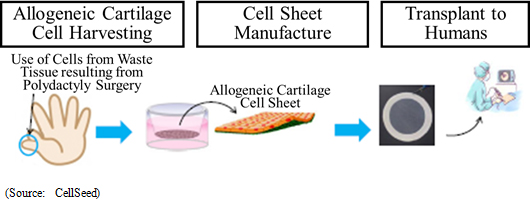 <Overseas Business Deployment>
A contract was signed in April 2017 allowing MetaTech (AP) Inc. (in New Taipei City, Taiwan, represented by Mr. Li San Hu. Hereafter referred to as MetaTech) to become the sole company conducting business deployment of the cell sheet regenerative medicine business (epithelial cell sheets for esophageal regeneration and regenerated cartilage sheet) in Taiwan (Discussions between the two companies were started in December 2016 and a ¥50 million derivation study deposit was paid by MetaTech to CellSeed).
(1) Business Alliance Contract in Taiwan  Contract Overview, Compensation
Going forward, MetaTech AP will promote development of regenerative cell sheet medicine in Taiwan with the support of CellSeed. According to the terms of the contract, CellSeed is expected to receive compensation amounting to ¥1.250 billion in accordance with the achievement of targets (Milestone income) in the development of epithelial cell sheets for esophageal regeneration and regenerated cartilage sheet and for data relating to development and manufacturing provided to MetaTech as support (Development support income). In addition, royalties of an unspecified percent of any products that are launched into the local market are also expected to be paid to CellSeed.
"Development Support Sales" from Fiscal Year December 2017
CellSeed expects to begin booking "development support sales" from the contract formed with MetaTech from fiscal year December 2017. In the event that these sales will have a large influence upon current year earnings, CellSeed will make efforts to disclose information regarding the impact of these sales in a timely fashion. Because MetaTech is expected to start development within fiscal year 2017, development support sales are expected to be booked. However, milestone income, which will be paid in line with progress of the development, is expected to begin being booked from the coming fiscal year and for the coming three to four years. While regenerative medicine products are subject to the normal medical approval process, considerations are being made for expedited approval in Taiwan.
MetaTech Overview
MetaTech is primarily a wholesaler and retailer of electronic materials, medical and beauty products, and medical equipment, and is a publicly traded company listed on the Over the Counter Market in Taiwan providing biotechnology and other services there. MetaTach was established on September 17, 1998, and boasted of sales, recurring income, total assets, net assets, and capital of Taiwan $2.153 billion, $6 million, $1.033 billion, $524 million and $4 million respectively (At an exchange rate of ¥3.65 per Taiwan Dollar: ¥7.858, ¥0.022, ¥3.770, ¥1.913 and ¥0.160 billion respectively).
(2) United States BIO International Convention Participation
CellSeed participated in the BIO International Convention held in San Diego, California from June 17 to 20, 2017. Companies ranging from major corporations to startup companies in the biotechnology related realm participated in this convention, with 1,800 companies displaying booths, 4,000 companies participating and 16,000 people visiting the Convention. The pre-registration system for pre-matching using partnering function, and interview system during the course of the event are unique features of this Convention. CellSeed operated a booth at this Convention with the goal of surveying and finding candidates for collaborative work on its cell sheet regenerative medicine business in the future, and conducted interviews with multiple pharmaceutical companies, US State Ministries of Commerce, etc. Currently, CellSeed is conducting follow-up deliberations with the companies it interviewed during the Convention.
Funding
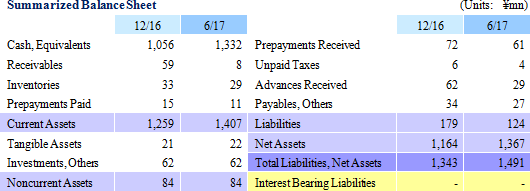
CellSeed conducted a third party placement of options for new shares (16th) on March 6, 2017 (A commitment issue which requires the options to be exercised within a certain amount of time). As of end July 2017, 59% of the options had been exercised for a total of 13.00 million shares (The first half of the commitment to exercise shares completed), contributing to total cash and deposits of ¥1.332 billion recorded at the end of the current first half. CellSeed now expects to be able to secure funding needed to be used for the acquisition of sales approval for "epithelial cell sheets for esophageal regeneration" due to its outlook for all of the options for new shares to be exercised by May 14, 2018.
Options for New Shares Overview
 |
||||||||||||||||||||
| Future Highlights |
|
|
| <Reference: CellSeed's corporate governance> |
 ◎Corporate Governance Report
Latest Update: April 5, 2017
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people's health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more. <Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)> CellSeed has stated, "Our company implements all the basic principles stipulated in the Corporate Governance Code as a JASDAQ listing company." Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2018 Investment Bridge Co., Ltd. All Rights Reserved. |





