| CellSeed Inc. (7776) |
|
||||||||
Company |
CellSeed Inc. |
||
Code No. |
7776 |
||
Exchange |
JASDAQ |
||
Industry |
Precision Instrument (Manufacturing) |
||
President |
Setsuko Hashimoto, Ph.D. |
||
HQ Address |
Telecom Center Building, Aomi 2-5-10, Koto-ku, Tokyo |
||
Year-end |
December |
||
URL |
|||
* Stock price as of closing on August 24, 2018. Number of shares at the end of the most recent quarter excluding treasury shares. ROE and BPS are the actual values of the previous term.
|
||||||||||||||||||||||||
|
|
* Estimates are those of the company. From FY12/16, the definition of net income has been changed to net income attributable to the parent company shareholders (the same shall apply hereinafter).
This Bridge Report presents the first half of fiscal year 2018 earnings results and the future outlook of CellSeed Inc. |
| Key Points |
 |
| Company Overview |
|
CellSeed uses the fundamental technologies of "cell sheet engineering" developed in Japan by Professor Okano of the Tokyo Women's Medical University in its "cell sheet regenerative medicine" that employs "cell sheets" for the cell regenerative medicine business, and the regenerative medicine support business, where temperature responsive cell cultureware used to fabricate cell sheets are developed and sold and the regenerative medicine consignment services, which support for research and development and commercialization of regenerative medicine, is provided.
Cell sheet regenerative medicine business
Commercialization through the cultivation of new businesses is being conducted in joint clinical research efforts with universities. The current development pipeline consists of the two main realms of "epithelial cell sheet for esophageal regeneration" based upon the fundamental technology of "regenerative cell sheet engineering", and "regenerated cartilage sheet" of knee osteoarthritis. Case registrations for "epithelial cell sheet for esophageal regeneration" have been completed, and CellSeed is conducting preparations for the submission of sales approval application and hopes to gain sales approval within fiscal year December 2019. Also, the collaborative business partner in Taiwan is to submit clinical trial approval application. Preparations for the start of clinical trials within Japan for "regenerated cartilage sheet" are also being promoted. However, regenerative medicine products using "cell sheet engineering" are not only limited to "epithelial cell sheet for esophageal regeneration" and "regenerated cartilage sheets". Clnical research is being promoted for other treatments including cornea, periodontium, middle ear, lung, heart, liver and pancreas applications, and clinical data is already available for these applications. In Europe, "periodontal tissue regenerative sheet" and "epithelial cell sheet for esophageal regeneration" products are currently being considered as next candidates for development pipelines. Development of various applications is expected to begin once preparations made with research institutions in each respective product development realm and geographic region are completed.
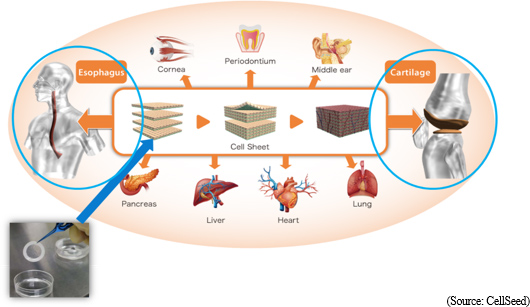 Regenerative medicine supporting business
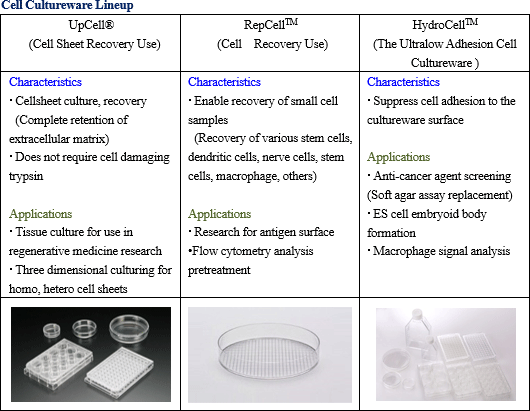
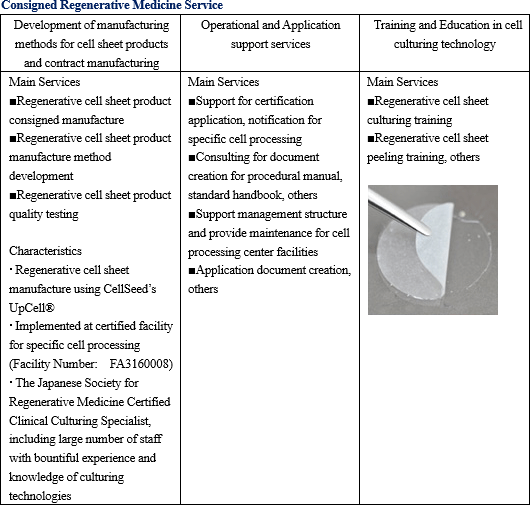
Consigned regenerative medicine services for the comprehensive support regarding regenerative medicine and development, manufacture, and sales of temperature responsive cell cultureware are being conducted. The main services provided within the consigned regenerative medicine services include regenerative cell sheet product manufacturing method development, consigned manufacturing, operational and application support, cell culturing technician training and others. Regenerative medicine supporting business
Consigned regenerative medicine services for the comprehensive support regarding regenerative medicine and development, manufacture, and sales of temperature responsive cell cultureware are being conducted. The main services provided within the consigned regenerative medicine services include regenerative cell sheet product manufacturing method development, consigned manufacturing, operational and application support, cell culturing technician training and others.
  Cell Processing Facility (CPF)
This Cell Processing Facility boasts of 763 square meters of floor space, and has an automated monitoring system to control cleanliness, room pressure, temperature and humidity, Operational status of equipment (Incubator, Reagent stocker etc.) and a surveillance camera system throughout the entire facility. In addition, this facility is only 20 minutes drive from Haneda International Airport. In March 2017, "manufacture and process specified cell products " in accordance with Article 35, paragraph 1 of the Act on Safety of Regenerative Medicine was granted by the Ministry of Health, Labour and Welfare. Consequently, CellSeed is able to provide consigned processing business for cell sheets. Cell Processing Facility (CPF)
This Cell Processing Facility boasts of 763 square meters of floor space, and has an automated monitoring system to control cleanliness, room pressure, temperature and humidity, Operational status of equipment (Incubator, Reagent stocker etc.) and a surveillance camera system throughout the entire facility. In addition, this facility is only 20 minutes drive from Haneda International Airport. In March 2017, "manufacture and process specified cell products " in accordance with Article 35, paragraph 1 of the Act on Safety of Regenerative Medicine was granted by the Ministry of Health, Labour and Welfare. Consequently, CellSeed is able to provide consigned processing business for cell sheets.
 |
| First Half of Fiscal Year 2018 Earnings Results and Full-Year Estimates |
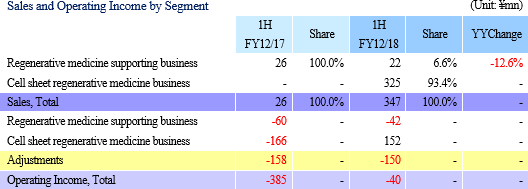
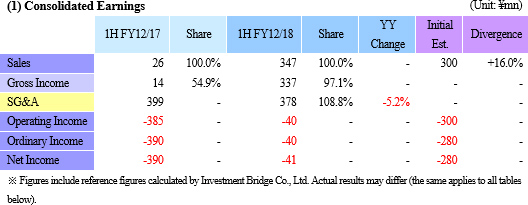 Sales and Operating Loss of ¥347 and ¥40 Million Booked (¥26 and ¥385 Million in Previous Term)
Regenerative medicine support business saw similar levels of sales as the previous term (¥22 million), but the provision of development data to the collaborative partner in Taiwan MetaTech Inc. allowed the cell sheet regenerative medicine business to book ¥325 million in sales (Data provision was completed ahead of schedule allowing sales to exceed initial estimates).
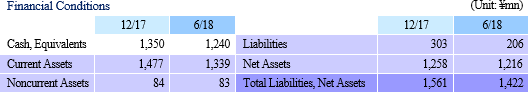
While the higher sales allowed gross income to grow, the delays in booking of maintenance fees for the cell processing facility (Regular maintenance) and some of research and development expenses (Down 11.6% year-on-year to ¥158 million) into the second half of the fiscal year allowed sales, general and administrative expenses to decline. Consequently, the operating loss declined from ¥385 million in the pervious first half to ¥40 million in the current first half. Clinical trial case registrations for epithelial cell sheet for esophageal regeneration, which have been conducted since August 2016, were completed in April. Sales and Operating Loss of ¥347 and ¥40 Million Booked (¥26 and ¥385 Million in Previous Term)
Regenerative medicine support business saw similar levels of sales as the previous term (¥22 million), but the provision of development data to the collaborative partner in Taiwan MetaTech Inc. allowed the cell sheet regenerative medicine business to book ¥325 million in sales (Data provision was completed ahead of schedule allowing sales to exceed initial estimates).
While the higher sales allowed gross income to grow, the delays in booking of maintenance fees for the cell processing facility (Regular maintenance) and some of research and development expenses (Down 11.6% year-on-year to ¥158 million) into the second half of the fiscal year allowed sales, general and administrative expenses to decline. Consequently, the operating loss declined from ¥385 million in the pervious first half to ¥40 million in the current first half. Clinical trial case registrations for epithelial cell sheet for esophageal regeneration, which have been conducted since August 2016, were completed in April.
    |
| Medium Term Business Plan (Fiscal Years December 2018 to 2020) |
|
<Key Points>
 Obtain approval and start sales for epithelial cell sheet for esophageal regeneration Obtain approval and start sales for epithelial cell sheet for esophageal regeneration
 Accelerate development of regenerated cartilage sheet Accelerate development of regenerated cartilage sheet
 Start development of the next generation product Start development of the next generation product
 Establish a supply chain system Establish a supply chain system
 Develop new regenerative medicine supporting products and cultivate earnings opportunities Develop new regenerative medicine supporting products and cultivate earnings opportunities
 Promote business alliance for global expansion
Obtain Approval and Start Sales for Epithelial Cell Sheet for Esophageal Regeneration, Establish Supply Chain System
Esophageal Cancer in Japan
According to CellSeed, 22,000 patients within Japan are diagnosed with esophageal cancer every year (90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma), with 11,500 patients dying every year. In addition, the rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients, with five year survival rates for males and females said to be 36% and 44%, respectively. The endoscopic resection surgery (ESD) was posted in the drug price list from 2008 and is on the rise (about 20% of patients diagnosed with esophageal cancer undergo surgery), but its side effect of esophageal stricture after surgery has been recognized as a problem.
Obtain Approval and Start Sales for Epithelial Cell Sheet for Esophageal Regeneration, Establish Supply Chain System
To treat esophageal tear prevent stricture , Advanced Biomedical Science Institute of Tokyo Women's Medical University (TWMU) developed regenerative medicine for esophageal cancer, using epithelial cell sheet for esophageal regeneration. Producing cell sheets by culturing the cells, taken from patient's oral mucosa, in temperature-responsive cell cultureware for 2 weeks. It can prevent esophageal stricture by transplanting cell sheets to esophageal tumor after the excision of esophageal cancer in endoscopic surgery.
There have been 30 clinical cases from 2008 to 2014, 10 cases at TWMU, 10 cases at TWMU and Nagasaki University (Long-distance transportation test: Collect cells at Nagasaki University, Culture at TWMU, Transplant at Nagasaki University), and 10 cases at Karolinska University Hospital (Sweden). CellSeed has concluded Basic Agreement for Development with TWMU and has taken over the research results. Promote business alliance for global expansion
Obtain Approval and Start Sales for Epithelial Cell Sheet for Esophageal Regeneration, Establish Supply Chain System
Esophageal Cancer in Japan
According to CellSeed, 22,000 patients within Japan are diagnosed with esophageal cancer every year (90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma), with 11,500 patients dying every year. In addition, the rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients, with five year survival rates for males and females said to be 36% and 44%, respectively. The endoscopic resection surgery (ESD) was posted in the drug price list from 2008 and is on the rise (about 20% of patients diagnosed with esophageal cancer undergo surgery), but its side effect of esophageal stricture after surgery has been recognized as a problem.
Obtain Approval and Start Sales for Epithelial Cell Sheet for Esophageal Regeneration, Establish Supply Chain System
To treat esophageal tear prevent stricture , Advanced Biomedical Science Institute of Tokyo Women's Medical University (TWMU) developed regenerative medicine for esophageal cancer, using epithelial cell sheet for esophageal regeneration. Producing cell sheets by culturing the cells, taken from patient's oral mucosa, in temperature-responsive cell cultureware for 2 weeks. It can prevent esophageal stricture by transplanting cell sheets to esophageal tumor after the excision of esophageal cancer in endoscopic surgery.
There have been 30 clinical cases from 2008 to 2014, 10 cases at TWMU, 10 cases at TWMU and Nagasaki University (Long-distance transportation test: Collect cells at Nagasaki University, Culture at TWMU, Transplant at Nagasaki University), and 10 cases at Karolinska University Hospital (Sweden). CellSeed has concluded Basic Agreement for Development with TWMU and has taken over the research results.
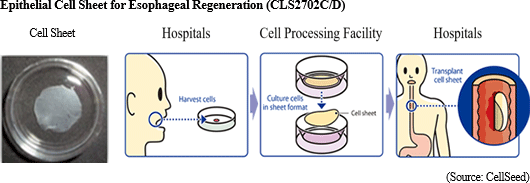 Clinical trial case registrations were completed during the second quarter of fiscal year December 2018 (April 2018), with sales approval application expected to be submitted during the first half of fiscal year December 2019. CellSeed received the "Sakigake Designation Scheme" in February 2017, so is expected to be able to receive sales approval within about six months after application is submitted. Therefore, sales approval is expected to be granted and sales are expected to begin within fiscal year December 2019 (After the National Health Insurance Price Listing is completed), with full scale sales expected to be conducted in fiscal year December 2020. In the near term, establishment of a supply chain ranging from hospitals for oral muscosa membrane sample acquisition, to culturing facility for cell sheet cultivation, and to hospitals for cell sheet transplant is promoted.
In Europe, corporate led clinical trials are being considered in Sweden led by the subsidiary CellSeed Sweden AB, established in May 2015, and deliberations with the European Medicines Agency (EMA) began in 2016 for approval throughout all of Europe. However, the Company is considering commercialization of cell sheet regenerative medicine business in Japan as the top priority of its product development plans, so development of epithelial cell sheet for esophageal regeneration in Europe is considered as a "next generation product" candidate.
As previously explained, the provision of development data to the collaborative partner in Taiwan MetaTech is proceeding smoothly, with MetaTech expected to submit clinical trial notifications within 2018.
Accelerate Development of Regenerated Cartilage Sheet
Regenerated cartilage sheet is the result of research conducted jointly by CellSeed and Professor Masato Sato of Tokai University School of Medicine Department of Orthopedics, and is a treatment designed to be used on patients with damaged and deformed cartilage caused by injuries from sports and osteoarthritis due to aging. Although there are no effective treatments currently available for them, research is being conducted jointly with Professor Sato aiming for the fundamental regeneration of cartilage surface. Knee cartilage is known as "glass cartilage" and differs from cartilage of the ear and nose in its superior cushioning and wear functions, which makes its regeneration difficult. However, regeneration of "glass cartilage" has been confirmed in joint clinical research using "regenerated cartilage sheet."
Based upon the fundamental agreement for development formed with Tokai University, efforts are being promoted to achieve commercialization with strategy negotiations being conducted with the Pharmaceuticals and Medical Devices Agency (PMDA, conducts approval examination), and the Japan Agency for Medical Research and Development (AMED, conducts regenerative medicine research and development) for the leveraging of business in development. In addition, partial development data is being provided to MetaTech of Taiwan during fiscal year December 2018 to help it achieve product commercialization.
According to CellSeed, knee osteoarthritis is a refractory disease where articular cartilage degeneration progresses slowly and the number of patients over the age of 40 within Japan is estimated to be 25.30 million, of which some 8.00 million are estimated to have active symptoms (survey conducted by Tokyo University School of Medicine). In addition, the number of patients is expected to increase due to Japan's aging society, and therefore it is considered a disease that needs to be handled urgently from the aspects of health and longevity of the Japanese, and nursing and medical care expenses.
Clinical trial case registrations were completed during the second quarter of fiscal year December 2018 (April 2018), with sales approval application expected to be submitted during the first half of fiscal year December 2019. CellSeed received the "Sakigake Designation Scheme" in February 2017, so is expected to be able to receive sales approval within about six months after application is submitted. Therefore, sales approval is expected to be granted and sales are expected to begin within fiscal year December 2019 (After the National Health Insurance Price Listing is completed), with full scale sales expected to be conducted in fiscal year December 2020. In the near term, establishment of a supply chain ranging from hospitals for oral muscosa membrane sample acquisition, to culturing facility for cell sheet cultivation, and to hospitals for cell sheet transplant is promoted.
In Europe, corporate led clinical trials are being considered in Sweden led by the subsidiary CellSeed Sweden AB, established in May 2015, and deliberations with the European Medicines Agency (EMA) began in 2016 for approval throughout all of Europe. However, the Company is considering commercialization of cell sheet regenerative medicine business in Japan as the top priority of its product development plans, so development of epithelial cell sheet for esophageal regeneration in Europe is considered as a "next generation product" candidate.
As previously explained, the provision of development data to the collaborative partner in Taiwan MetaTech is proceeding smoothly, with MetaTech expected to submit clinical trial notifications within 2018.
Accelerate Development of Regenerated Cartilage Sheet
Regenerated cartilage sheet is the result of research conducted jointly by CellSeed and Professor Masato Sato of Tokai University School of Medicine Department of Orthopedics, and is a treatment designed to be used on patients with damaged and deformed cartilage caused by injuries from sports and osteoarthritis due to aging. Although there are no effective treatments currently available for them, research is being conducted jointly with Professor Sato aiming for the fundamental regeneration of cartilage surface. Knee cartilage is known as "glass cartilage" and differs from cartilage of the ear and nose in its superior cushioning and wear functions, which makes its regeneration difficult. However, regeneration of "glass cartilage" has been confirmed in joint clinical research using "regenerated cartilage sheet."
Based upon the fundamental agreement for development formed with Tokai University, efforts are being promoted to achieve commercialization with strategy negotiations being conducted with the Pharmaceuticals and Medical Devices Agency (PMDA, conducts approval examination), and the Japan Agency for Medical Research and Development (AMED, conducts regenerative medicine research and development) for the leveraging of business in development. In addition, partial development data is being provided to MetaTech of Taiwan during fiscal year December 2018 to help it achieve product commercialization.
According to CellSeed, knee osteoarthritis is a refractory disease where articular cartilage degeneration progresses slowly and the number of patients over the age of 40 within Japan is estimated to be 25.30 million, of which some 8.00 million are estimated to have active symptoms (survey conducted by Tokyo University School of Medicine). In addition, the number of patients is expected to increase due to Japan's aging society, and therefore it is considered a disease that needs to be handled urgently from the aspects of health and longevity of the Japanese, and nursing and medical care expenses.
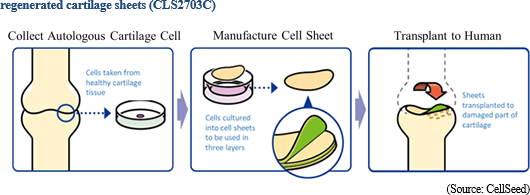 Research and development for patients' own cells and allogeneic cells are being promoted, with eight cases of clinical research for regenerated cartilage sheet using patients' own cells being conducted by Tokai University and the same university expected to submit application for this treatment as an advanced medical treatment. Preparations for applications have been promoted based upon discussions with the Ministry of Health, Labor and Welfare, and are expected to be considered by the Advanced Medical Treatment Committee after application has been submitted. Based upon the outcome of consideration as an advanced medical treatment, corporate clinical trials are expected to be implemented. And if this treatment is granted advanced medical treatment status, it will be recognized as a treatment, which can be used together with other treatments eligible for medical health insurance despite being excluded from the public medical health insurance system. Furthermore, it has already been decided that CellSeed will be compensated for consigned cell sheet processing for use in advanced medical treatments.
One case of transplant of regenerated cartilage sheet using allogeneic cells has been implemented in February 2017 by Tokai University with favorable results. Ten patients are expected to receive transplants as part of the clinical research expected to be conducted over the next three years, with the bulk of cases expected to be implemented in fiscal year December 2019 (Three cases have been implemented in the first half of fiscal year December 2018). During this time, CellSeed will promote clinical trial preparations, regulatory science strategical consultations and comprehensive regulatory science consultations with a goal of starting corporate clinical trials within fiscal year December 2020.
Status of Business Collaboration with MetaTech of Taiwan
CellSeed granted MetaTech Inc. the sole development, manufacture and sales rights in Taiwan for its cell sheet regenerative medicine business (Epithelial cell sheet for esophageal regeneration, regenerated cartilage sheet) in April 2017. According to the agreement between the two companies, CellSeed will receive a maximum of ¥1.250 billion as milestone income, development and manufacture related data provision fees, and development support fees to support MetaTech's deployment of CellSeed's business in Taiwan. Furthermore, MetaTech has agreed to also pay CellSeed royalty in accordance with sales of any resulting products in the Taiwan market.
As explained previously, provision of partial development data has progressed at a faster than expected pace during the first half of fiscal year December 2018, and this earlier than expected provision of this data has resulted in the payment of ¥325 million during the first half. MetaTech is promoting preparations for clinical trial notification submission for epithelial cell sheet for esophageal regeneration during fiscal year December 2018. Revisions to laws relating to cell treatments, which are considered to be advanced medical treatments, in Taiwan (Expected to be implemented in September 2018) are being promoted and CellSeed's regenerated cartilage sheet is expected to be included in this category.
Promotion of Collaborative Arrangements for Deployment of Business Globally
CellSeed will promote support for its collaborative business partner MetaTech, while at the same time searching for other business partners in various countries of Asia, Europe and North America. As part of this strategy, CellSeed has participated in six events during the first half of the fiscal year, and expects to participate in two more events in August.
Research and development for patients' own cells and allogeneic cells are being promoted, with eight cases of clinical research for regenerated cartilage sheet using patients' own cells being conducted by Tokai University and the same university expected to submit application for this treatment as an advanced medical treatment. Preparations for applications have been promoted based upon discussions with the Ministry of Health, Labor and Welfare, and are expected to be considered by the Advanced Medical Treatment Committee after application has been submitted. Based upon the outcome of consideration as an advanced medical treatment, corporate clinical trials are expected to be implemented. And if this treatment is granted advanced medical treatment status, it will be recognized as a treatment, which can be used together with other treatments eligible for medical health insurance despite being excluded from the public medical health insurance system. Furthermore, it has already been decided that CellSeed will be compensated for consigned cell sheet processing for use in advanced medical treatments.
One case of transplant of regenerated cartilage sheet using allogeneic cells has been implemented in February 2017 by Tokai University with favorable results. Ten patients are expected to receive transplants as part of the clinical research expected to be conducted over the next three years, with the bulk of cases expected to be implemented in fiscal year December 2019 (Three cases have been implemented in the first half of fiscal year December 2018). During this time, CellSeed will promote clinical trial preparations, regulatory science strategical consultations and comprehensive regulatory science consultations with a goal of starting corporate clinical trials within fiscal year December 2020.
Status of Business Collaboration with MetaTech of Taiwan
CellSeed granted MetaTech Inc. the sole development, manufacture and sales rights in Taiwan for its cell sheet regenerative medicine business (Epithelial cell sheet for esophageal regeneration, regenerated cartilage sheet) in April 2017. According to the agreement between the two companies, CellSeed will receive a maximum of ¥1.250 billion as milestone income, development and manufacture related data provision fees, and development support fees to support MetaTech's deployment of CellSeed's business in Taiwan. Furthermore, MetaTech has agreed to also pay CellSeed royalty in accordance with sales of any resulting products in the Taiwan market.
As explained previously, provision of partial development data has progressed at a faster than expected pace during the first half of fiscal year December 2018, and this earlier than expected provision of this data has resulted in the payment of ¥325 million during the first half. MetaTech is promoting preparations for clinical trial notification submission for epithelial cell sheet for esophageal regeneration during fiscal year December 2018. Revisions to laws relating to cell treatments, which are considered to be advanced medical treatments, in Taiwan (Expected to be implemented in September 2018) are being promoted and CellSeed's regenerated cartilage sheet is expected to be included in this category.
Promotion of Collaborative Arrangements for Deployment of Business Globally
CellSeed will promote support for its collaborative business partner MetaTech, while at the same time searching for other business partners in various countries of Asia, Europe and North America. As part of this strategy, CellSeed has participated in six events during the first half of the fiscal year, and expects to participate in two more events in August.
 |
| Future Highlights |
|
Within Japan, case registrations of epithelial cell sheet for esophageal regeneration treatments conducted since August 2016 have been completed. And in overseas markets, data provision to MetaTech is proceeding smoothly and will continue going forward. Some of the data provided is for regenerated cartilage sheets and further sales are expected to be booked during the fourth quarter of the current fiscal year. Sales approval application submission is expected to be completed by this time next year, with full scale sales of epithelial cell sheet for esophageal regeneration expected to begin from fiscal year December 2020. In addition to plans to submit clinical trial notifications for epithelial cell sheet for esophageal regeneration in Taiwan within fiscal year December 2018, revisions to laws relating to cell treatments are expected to act as a tailwind for regenerated cartilage sheets. While a "wait and see" stance regarding the near term business conditions, may be warranted, there appears to be few issues with funding for the business for the foreseeable future. Consequently, CellSeed's business can be expected to change dramatically around 2025 due to the contributions of epithelial cell sheet for esophageal regeneration and regenerated cartilage sheets.
|
| <Reference: CellSeed's corporate governance> |
 ◎Corporate Governance Report
Latest Update: April 5, 2017
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people's health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.
In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)>
CellSeed has stated, "Our company implements all the basic principles stipulated in the Corporate Governance Code as a JASDAQ listed company." ◎Corporate Governance Report
Latest Update: April 5, 2017
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people's health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.
In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)>
CellSeed has stated, "Our company implements all the basic principles stipulated in the Corporate Governance Code as a JASDAQ listed company."
Disclaimer
This report is intended solely for information purposes and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the company and obtained from sources that we judge to be reliable. However, we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2018 Investment Bridge Co., Ltd. All Rights Reserved. |



