Bridge Report:(4549)EIKEN CHEMICAL the fiscal year March 2022
 President Tsugunori Notomi | EIKEN CHEMICAL CO.,LTD.(4549) |
|
Company Information
Exchange | TSE Prime |
Industry | Pharmaceuticals (manufacturing and sales) |
President | Tsugunori Notomi |
HQ Address | 7 Yamaguchi building, 4-19-9 Taito, Taito-ku, Tokyo 110-8408, Japan |
Year-end | End of March |
Homepage |
Stock Information
Share Price | Share Outstanding | Market Cap. | ROE (Act.) | Trading Unit | |
1,613yen | 43,541,438 Shares | 70,232 million yen | 14.3% | 100 Shares | |
DPS (Est.) | Dividend Yield (Est.) | EPS (Est.) | PER (Est.) | BPS (Act.) | PBR (Act.) |
35.00 | 2.2% | 97.66 yen | 16.5 times | 1,230.55 yen | 1.3 times |
* Share price is as of the end of June 20. All figures are from the FY March 2022 financial settlement report.
Business Performance Trends
Fiscal Year | Net Sales | Operating Income | Ordinary Income | Net Income | EPS | DPS |
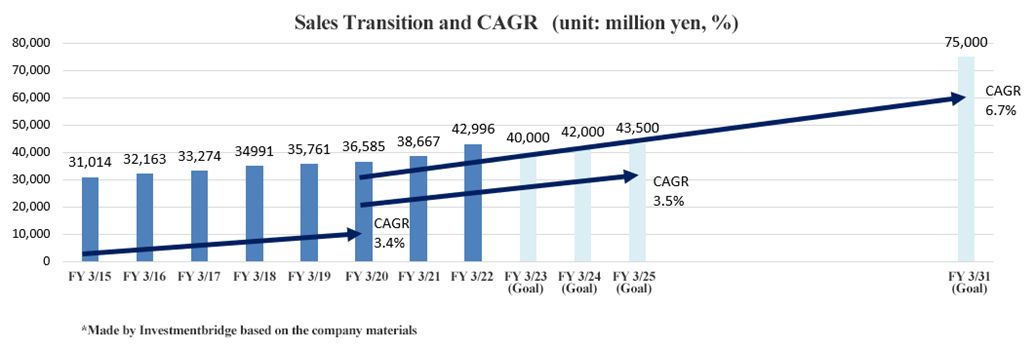
March 2019 Act. | 35,761 | 4,611 | 4,681 | 3,447 | 93.63 | 30.00 |
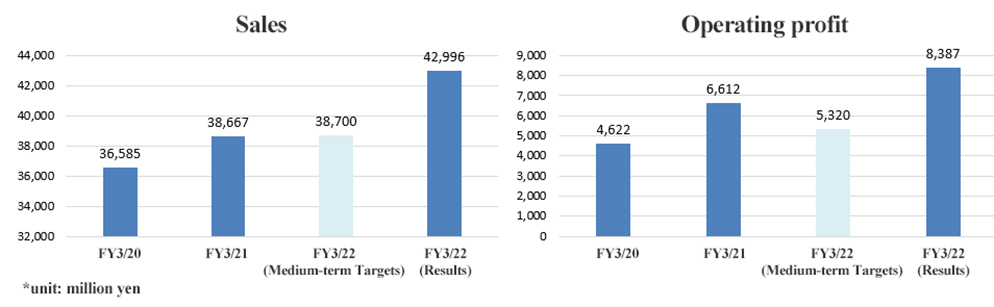
March 2020 Act. | 36,585 | 4,622 | 4,723 | 3,538 | 95.95 | 30.00 |
March 2021 Act. | 38,667 | 6,612 | 6,808 | 5,044 | 136.65 | 41.00 |
March 2022 Act. | 42,996 | 8,387 | 8,508 | 6,218 | 168.28 | 51.00 |
March 2023 Est. | 40,000 | 4,540 | 4,570 | 3,610 | 97.66 | 35.00 |
* Unit: Million-yen, Yen. The definition for net income means net income attributable to owners of parent.
This Bridge Report presents EIKEN CHEMICAL’s earnings results for the fiscal year March 2022 and New Mid-Term Management Plan etc.
Table of Contents
Key Points
1. Company Overview
2. Fiscal Year Ended March 2022 Earnings Results
3. Fiscal Year Ending March 2023 Earnings Estimates
4. “EIKEN ROAD MAP 2030” and New Medium-Term Management Plan
5. Interview with President Notomi
6. Conclusions
<Reference : Regarding Corporate Governance>
Key Points
- In the fiscal year (FY) 2022, sales grew 11.2% year on year to 42.9 billion yen. Demand for detection reagents increased due to the resumption of various screenings/screening programs, the recovery trend in the number of outpatients, and the spread of COVID-19 in the fourth quarter. Overseas sales increased significantly mainly for fecal immunochemical test reagents. Operating income increased 26.8% year on year to 8.3 billion yen. Gross profit, which was mainly from COVID-19 detection reagents, increased 11.0% year on year. On the other hand, sales expenses increased slightly as sales activities had to be restrained due to COVID-19.
- With the rebound in infection rate in Japan after July, demand for COVID-19 detection reagents exceeded expectation while expenses were lower than expected, so both sales and profit exceeded the initial forecasts. In response to the favorable business performance, the year-end dividend was increased by 10 yen/share to 31 yen/share, and the dividend for FY 2022 was 51 yen/share, up 10 yen/share from the previous fiscal year. The payout ratio was 30.3%.
- For FY 2023, sales are expected to decrease 7.0% year on year to 40 billion yen, and operating income is expected to decrease 45.9% year on year to 4,540 million yen. Demand for genetic testing for COVID-19 infections (COVID-19 detection reagents) is expected to decrease significantly from the previous fiscal year. The company expects a decrease in profit due to increased expenses toward the investment in R&D and the development of the management base, in addition to a decrease in sales of highly profitable COVID-19 detection reagents and patent rights income under the LAMP method. The dividend is expected to be 35 yen/share, down 16 yen/share from the previous fiscal year. The expected dividend payout ratio is 35.8%.
- To respond to the unfolding changes in its business environment and operate form a perspective of sustainability management, the company revamped its existing management framework “EIKEN ROAD MAP 2019” and redefined it as “EIKEN ROAD MAP 2030” with 2030 as the target year for its fulfillment. In addition, the company formulated its first Medium-Term Management Plan to achieve its goals. While the existing business domain remains as its core businesses, the company has also set three area as key business fields: (i) Contribution to cancer prevention and treatment, (ii) Contribution to the eradication and control of infectious diseases, and (iii) Provision of products and services useful for health care.
- The company predicts a decrease in both sales and profit in the current fiscal year. The demand for COVID-19 detection reagents, which had been increasing significantly in the previous two fiscal years, will be unavoidably on a reactionary decline from this fiscal year onward due to the subsiding infection rate. However, when comparing the CAGR (Compound Annual Growth Rate) for the five years before COVID-19 pandemic and the five years until the final fiscal year of the post-pandemic Medium-Term Management Plan, there is no significant difference between the two of them, as both are just over 3%.
- On the other hand, as COVID-19 pandemic subsides, the number of conventional tests and medical inspections is on a recovery trend, and the growth of fecal immunochemical test reagents overseas in particular has been remarkable. Although the stock price has been on a downward trend since the beginning of this fiscal year in response to the forecasts for the current fiscal year, it should be noted that there has been no significant change in the speed of the company's growth, and it is also planning to accelerate the growth speed thereafter. We look forward to witnessing how the company will progress through the various measures to realize its visions set out in EIKEN ROAD MAP 2030.
1. Company Overview
EIKEN CHEMICAL is a general manufacturer of clinical diagnostics, including immunological and serological, microbiological, clinical chemistry, urine analysis and genetic screening test. It also develops and sells medical devices.
It offers many products that occupy high market share including fecal immunochemical test that occupy about 60% of the domestic share, Urinalysis test, Microbiological test and so on. Its unique gene amplification technology, “LAMP”, is recognized in the world. With the fecal immunochemical test reagents, urinalysis test strips and LAMP, EIKEN is aiming to become a global corporation.
1-1 History
Founded as Koa Kagakukogyo Co., Ltd. in 1939, the company started manufacturing and selling nutritional foods and pharmaceuticals made from livestock organs. In 1949, it was the first company in Japan to successfully commercialize a powdered agar for the detection of bacteria (Salmonella-Shigella [SS] agar). In 1961, it established the Clinical Diagnostics Division and began R&D on in-vitro diagnostics.
In 1989, the company launched “OC-sensor,” the world's first fully automated fecal occult blood analyzer. This led to the establishment of its current overwhelming lead in this field.
After that, while expanding its business domains such as reagents for urinalysis testing and microbiological testing, in 1998, the company developed the LAMP method, a new gene amplification technology. It has launched various products using the LAMP method that are simpler, faster, and more accurate than conventional testing methods.
In 2005, the company concluded a joint development contract with FIND (Foundation for Innovative New Diagnostics) for a rapid genetic tuberculosis detection method, based on the LAMP method. It then proceeded with joint development related to testing for malaria, HIV, etc.
In March 2020, the company released COVID-19 detection reagent utilizing the LAMP method to be used against COVID-19, which has been spreading worldwide.
*For further information about the LAMP method and FIND, please refer to “2. Characteristics and Strengths (4) Competitive Advantages of the LAMP Method.”
1-2 Management Philosophy
“Management Philosophy”: Protect the health of the public through health care services.
“Management Vision”: EIKEN group is dedicated to leveraging expertise as a medical testing pioneer to increase corporate value by protecting the health of the public with products and services that customers can trust.
“Motto”: We EIKEN provide trustworthy quality and develop with technology.
EIKEN group formulates “EIKEN WAY” as its attitude toward each stakeholder, centering these philosophy vision, and motto.
(Source: EIKEN CHEMICAL)
1-3 Market Environment
Domestic Market
The market scale of clinical reagents is about 347 billion yen and 604.7 billion yen (including research reagents and diagnostic devices) as of 2020 (survey by the Japan Association of Clinical Reagents Industries, or JACRI. Eiken Chemical Data.).
To control rising medical costs, the Japanese government is focusing on preventive medicine such as special health check-ups (metabolic check-ups) and cancer screenings. It is expected that this, along with the aging population, will lead to an increase in the number of samples (number of specimens).
Some negative factors include the impact of population decline because of decreasing birth rates and revision of medical treatment fees (reduction). However, the trends of laboratory test fees which had been subject to revision of insurance (medical laboratory test fees) show that, even though they were cut by some 40% from 1997 to 2006, the fees have been stable or only slightly reduced after 2007. (Laboratory test fee in fiscal 2020: -1.12%)
This is a result of the activities for emphasizing the importance of prevention and checkups in the industry, including the company. In the medium term, the domestic market is expected to grow with an annual rate of around 2%.
Out of 139 member companies (as of April 2021) of JACRI mentioned above, about 80 are manufacturers, and there are about 15 companies with over 10 billion yen in sales. Most of them are small to medium sized companies. Because the test items of diagnostic tests range widely, each company has its own field of strength, and business segregation is already established in the industry. As a result, collaboration, such as supplying raw materials and products from other companies and manufacturing and selling them, is often observed. Against such a backdrop, the market is modestly growing. Therefore, there is currently no apparent trend of weeding out uncompetitive corporations.
Overseas Market
The global clinical laboratory test reagent/device market is estimated to be US$ 67.2 billion and, by region, the market is occupied by the USA at 36%, followed by Europe at 28% and Asia at 24% (As of 2018) (Fuji Keizai "2019 World Wide Clinical Testing Market" Eiken Chemical Co., Ltd.).
The overseas market is over ten times larger than the domestic market. In developed countries, the number of tests is increasing as aging of population progresses. Furthermore, in emerging countries, the needs for medical services are expanding because of economic and income growth. As a result, the annual growth rate of overseas market is expected to be over 5%, which is much higher than that of the domestic market. Therefore, the Japanese companies in the industry are vigorously undertaking globalization of their businesses.
In the global market, however, Roche being the most dominant with the sales of $61.4 billion in 2019, large global companies such as Abbott, SIEMENS, and Beckman are the main players, and in order to survive the competition, Japanese companies must strengthen their competitiveness by, for example, developing unique products or systems.
1-4 Business Description
1. What are Clinical Tests?
One type of clinical tests is the “Biological test” that directly examines the body using medical equipment such as X-ray, CT, MRI, electrocardiogram, and ultrasound. Another type of clinical tests is the “Laboratory test” that examines biological samples (specimens) obtained from people such as blood, urine/feces, and cells.
The clinical test reagents made by EIKEN CHEMICAL are the ones used for medical laboratory tests. For example, they are used to test infectious diseases or to measure small amounts of blood contained in stool. They are made to support diagnosis. Most of these reagents are called in vitro diagnostics (IVD) and are regulated by the Pharmaceutical and Medical Device Act so reagent manufacturers file applications with PMDA (Pharmaceuticals and Medical Devices Agency) and obtain its approval. Users include hospitals, clinics, medical offices, medical test centers that carry out tests commissioned by medical institutions, health screening centers, public health centers, and institutions for health research, and others.
2. Major Products
EIKEN CHEMICAL mainly manufactures and sells the following types of reagents and medical devices.
As they deal with a wide range of reagents, they not only sell their in-house products but also purchase and sell products from other companies.
Major in-house products include fecal immunochemical test reagents, microbiological reagents, immunological and serological reagents, urinalysis test strips, genetic testing reagents, etc. The sales ratio of in-house products to other companies’ products is approximately 60:40. The gross profit margin is approximately 55% for in-house products and approximately 35% for other companies’ products.
Product Name | Sales | Sales Proportion |
Fecal immunochemical test reagents (FIT) | 11,233 | 26.1% |
Immunological and serological reagents (excluding fecal immunochemical test reagents) | 9,359 | 21.8% |
Urinalysis test strips | 3,783 | 8.8% |
Microbiological test reagents | 3,924 | 9.1% |
Biochemical test reagents | 599 | 1.4% |
Equipment/Culture medium related to food and environmental | 2,252 | 5.2% |
Related molecular genetics (LAMP), (including its devices) | 7,445 | 17.3% |
Medical Devices (excluding molecular genetics related devices) | 4,396 | 10.2% |
Total sales | 42,996 | 100.0% |
*Results for the fiscal year ended March 2022. Unit: Million Yen
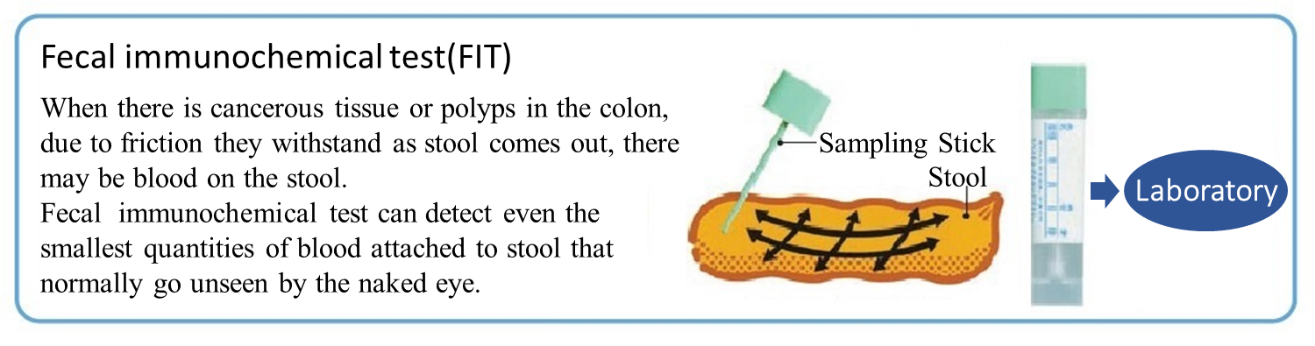
Fecal immunochemical test reagents
The major products for EIKEN CHEMICAL are reagents and sampling bottles for fecal immunochemical tests to specifically detect and measure human hemoglobin in feces as a colorectal cancer screening and diagnosis and are sold globally.
Immunological and serological reagents (excluding Fecal immunochemical test reagents)
EIKEN CHEMICAL develops, manufactures, and sells reagents for various tests, such as LZ Test EIKEN, a reagent for general-purpose automatic analyzers used for diagnosing rheumatism and inflammatory disorders and gastric cancer risk stratification test (the ABC method). The company also procures reagents for fully automated enzyme immunoassay devices and reagents for automatic glycohemoglobin analyzers from Tosoh Corporation, and sells them.
Urinalysis test strips
EIKEN CHEMICAL develops, manufactures, and sells “UROPAPER III ‘EIKEN’,” a urinalysis test strip for testing various items such as occult blood, protein and glucose, as well as the “UROPAPER III ‘EIKEN’,” a specialized test strip for fully automated urine analyzers.
Outside Japan, the company formed a business tie-up with Sysmex Corporation in 2017 and has sales.
Microbiological test reagents
Since its establishment, EIKEN CHEMICAL has been developing biological specimens as well as reagents for microbiological tests for food and environment in order to prevent infectious diseases and food poisoning. Currently, it develops, manufactures, and sells various reagents that are effective for diagnosis and treatment of microorganism infection, such as mediums, powder mediums, antimicrobial susceptibility tests, and rapid test reagents.
Clinical chemistry test reagents
EIKEN CHEMICAL develops, manufactures and sells reagents for clinical chemistry tests including “EXDIA XL ‘EIKEN’” series that assist to measure and analyze biological components in blood serum and urine, with a focus on the test items that are related to lifestyle related diseases.
Equipment/ Culture mediums related to food and environment
EIKEN CHEMICAL sells reagents for microbiological tests on food to detect food poisoning bacteria as well as reagents for environmental microbiological tests and equipment and devices to measure contamination of work environments.
Molecular genetics (LAMP)
In 1998, EIKEN CHEMICAL developed and patented an innovative gene amplification technology called “LAMP.” The LAMP is “simple, rapid, and accurate” and is a critical tool for Eiken’s future domestic and global expansion of its business. (Details are described below)
Medical devices
EIKEN CHEMICAL sells various types of automated analyzers. They contract manufacturing specialized equipment that uses their in-house reagent. Since beginning sales of “OC Sensor” in 1989, they have worked continuously on technological innovation and quality improvement of this fecal immunochemical test analyzer. Also, they offer the “US,” an automated urine analysis device that uses Eiken’s proprietary image processing system, the “BLEIA-1200,” a fully automated biochemistry photogenetic immunoassay device that was the world’s first of its kind in the clinical testing field, and “Loopamp EXIA,” a LAMP-based real time turbidity measuring device.
3. Sales structure
EIKEN CHEMICAL has 10 sales divisions in Japan. Its academic department supports sales promotion.
Out of 745 employees (consolidated) during FY 2022, about 300 belong to the sales department.
As for the sales channels for medical institutions such as hospitals, the Company’s direct sales partners are medical wholesale companies, and it has businesses with almost all the wholesale companies in the medical industry.
For overseas sales, EIKEN CHEMICAL has basically 1 agency per country, and the sales and maintenance are commissioned to the agencies.
EIKEN’s products are exported to 43 countries (FY 2021). The high proportion of overseas sales is occupied by the sales in the USA, Germany, Italy, Spain, England, France, Australia, South Korea, and Taiwan.
In addition to the Europe Branch in Amsterdam (the Netherlands), the Company is strengthening its manufacturing and sales structure through its consolidated subsidiary, “EIKEN CHINA CO., LTD.,” as well as aiming to expand its businesses by setting a business office in China. In the future, it will explore the possibility of making the office as a local corporation, as the size expands.
The overseas sales for FY 2022 are 8,868 million yen, out of which 5,184 million yen, 58.5%, is from the sales of fecal immunochemical test reagents.
1-5 ROE Analysis
| FY3/13 | FY3/14 | FY3/15 | FY3/16 | FY3/17 | FY3/18 | FY3/19 | FY3/20 | FY3/21 | FY3/22 |
ROE (%) | 10.9 | 8.3 | 8.3 | 8.9 | 10.0 | 8.3 | 10.3 | 9.9 | 12.9 | 14.4 |
Net Profit Margin | 8.56 | 6.61 | 6.77 | 7.55 | 8.77 | 7.45 | 9.64 | 9.67 | 13.04 | 14.46 |
Asset Turnover Ratio | 0.84 | 0.84 | 0.83 | 0.83 | 0.80 | 0.78 | 0.77 | 0.75 | 0.73 | 0.73 |
Leverage | 1.52 | 1.50 | 1.47 | 1.42 | 1.43 | 1.43 | 1.38 | 1.36 | 1.35 | 1.36 |
*Unit: %, times, x
In FY 2022, net profit margin continued to increase from the previous fiscal year due to the growth of performance of COVID-19 detection reagents and fecal immunochemical test reagents.
The company intends to continue fortifying priority measures, including developing high value-added products, creating new businesses and new markets, and improving profitability and productivity by reducing the COGS and SG&A ratios. By doing so, the company plans to maintain ROE above the cost of capital.
1-6 Characteristics and Strengths
(1) Products that Occupy High Share in the Market
The share of Eiken’s fecal immunochemical test reagents is ranked top (63%) in the domestic market. Furthermore, many of their in-house products occupy high market share in the market, for example, urinalysis test strips occupying approximately 27% (ranked second) of the market, and microbiological reagents occupying approximately 16% (ranked fourth) of the market.
The background to how Eiken’s fecal immunochemical test reagents have come to hold such a high share of the market includes that in 1987, Eiken began sales of “OC-Hemodia,” a visual determination method fecal immunochemical test reagents, a product that more closely conformed to user needs when compared to competitor’s products, and that in 1989 they adopted the latex photometric immunoassay method and began sales of “OC-Sensor,” the world’s first fully automated analyzer.
Also, the Health and Medical Service Act for the Aged was revised in 1992, making it possible to have fecal immunochemical test reagents as a method in colon cancer screening and diagnosis using public funds (no cost to the patient) which led to an accelerated spread and increased competition. But in 2001, Eiken began sales of the “OC-Sensor neo,” with completely remodeled functions, which increased its market share.
(Source: EIKEN CHRMICAL)
As for fecal immunochemical tests, Eiken will expand its business globally based on the above characteristics.
The immunochemical method used in Japan applies reagents that react only to human hemoglobin and can process a large volume simultaneously.
Meanwhile, in other countries, reagents for the chemical method (Guaiac method) based on old measuring principles are still used, which presents accuracy challenges. In 2011, the test guidelines in Europe have finally begun recommending automated analyzers that use the immunochemical method. As a result, the market is beginning to undergo a dramatic change.
Furthermore, although the chemical method is also still common in the United States, which has the largest potential market, trends show a gradual shift toward the immunochemical method. Additionally, new guidelines on colorectal cancer screening by USPSTF (US Preventive Medicine Special Committee) was published in June 2016. These guidelines pointed that the immunization method is superior to the conventional chemical method and pursuantly, and assessed Eiken’s fecal immunochemical test product, "the OC FIT-CHEK family of FITs" has the utmost inspection performance with high sensitivity and specificity. Besides, the large markets which are underdeveloping exist on the leading and emerging countries in Asia and South America.
Because the fecal immunochemical test market is a niche market, Japanese companies, the forerunners of the immunochemical method, own the most advanced technique, and hence Eiken’s reagents and equipment are the global standard.
(2) Focusing on research and development
EIKEN CHEMICAL is focusing on research and development of unique technologies as a research and development corporation, and the development of original products that respond to customers’ needs, using the unique technologies. The number of staff assigned for research and development is about 150.
The demand from the customers is higher quality of medicine. Specifically, they demand for higher differential diagnosis accuracy with high sensitivity and high quality and improved detection rate. In addition, easier usage will lead to reduction in the work of medical staff. Responding to such needs is critical.
Since its establishment in 1939, EIKEN CHEMICAL has accumulated unique technologies for manufacturing reagents. Their unique technologies are applied to the measuring principles of their devices such as fecal occult blood test analyzer, automated urine analyzer, and biochemiluminescent immunoassay analyzer “BLEIA” that are designed to optimize the performance of the reagents.
(3) Development of various types of products in various fields through alliance strategy
Because clinical test reagents have wide range of subjects and items, it is not possible for one company to develop, manufacture and sell all types of reagents. The other companies in the industry are focusing on the technologies and products that they are specialized in. However, as an integrated manufacturer of clinical test reagents, EIKEN CHEMICAL aims at stabilizing profit structure, expanding their own strengths through alliance strategy, and pursuing synergy effects such as complementing functions and acquiring new technologies, while dealing with a wide range of products and responding to the needs of customers and users such as medical institutions.
Another reason why they cover various types of products in various fields is that they believe that covering wide range of clinical tests is their social responsibility to protect the health of the public, as is stated in their management philosophy: “protect the health of the public through health care services”.
(4) Competitive Advantages of the “LAMP”
Thus, far the mainstream technology for amplifying genes as a process of gene tests has been what is called “PCR” Under such circumstances, in 1998, EIKEN CHEMICAL developed a unique technology called the “LAMP.”
Compared to the PCR, the “LAMP” offers the following superior characteristics and allows users to carry out simple, rapid, and accurate gene tests.
Simple | Amplification response occurs at a constant temperature (with the PCR, the temperature needs to be changed for amplification). |
Rapid | High amplification efficiency, with genes being detected within 30 to 60 minutes (with the general PCR, it takes 2 to 3 hours). |
Accurate | Extremely high specificity. |
Currently in the medical field, the LAMP is used to diagnose infectious diseases such as COVID-19, tuberculosis, mycoplasma (a genus of bacteria, it can also cause pneumonia), legionella, pertussis, etc.
EIKEN CHEMICAL is making focused efforts on infectious disease diagnostic test in order to establish the status of the LAMP. At the same time, it is promoting the use of the LAMP in other fields such as food production and processing, environment, agriculture/veterinary to spread and enhance recognition of the LAMP. In fact, the LAMP-based products have been commercialized one after another since 2002.
Furthermore, for the same purposes, EIKEN CHEMICAL is actively giving licenses to external companies to build the LAMP camp.
One of the major actions to spread the LAMP in the world is an alliance with “FIND.”
“FIND” stands for “Foundation for Innovative New Diagnostics” and is a non-profit organization recognized by the Swiss government, launched at a meeting of the United Nations World Health Assembly in May 2003. In its initial five years of existence, it received a grant from the Bill & Melinda Gates Foundation to start up their activities.
Their goal is to develop and introduce affordable, simple, and advanced diagnostic tests to eradicate infectious diseases in developing countries.
FIND’s scope of activities includes tuberculosis, malaria, and African sleeping disease. With tuberculosis, collaborative research between EIKEN CHEMICAL and FIND for a tuberculosis test using the LAMP began in July 2005. The purpose of this research is to improve the accuracy of tests by replacing the microscopy test (sputum smear test), which is the current practice in developing countries.
As a result of this collaboration, improvements which are not possible with the conventional PCR such as simplified pretreatment (PURE), improved reagents storage (store at room temperature) and simplified devices have been made to enable the developing countries to carry out the procedure (TB-LAMP).
This LAMP-based product was already launched in Japan in 2011.
After that, in order to obtain endorsement from the WHO (World Health Organization), FIND has completed its clinical evaluation in 14 developing countries and submitted this information to the WHO.
In consequence, the company has acquired the recommendation by WHO as an evaluation replaces with microscopic examination or as an inspection reinforcing microscopic examination in August 2016.
According to a report on global tuberculosis announced by WHO in November 2017, the number of patients suffering from tuberculosis in 202 countries all over the world in 2016 was 10.4 million, an increase of 0.8 million from 9.6 million in 2014. Additionally, the number of deceases was 1.7 million, an increase of 0.2 million from 1.5 million in 2014.
Most of them are inferred as matters of undiagnosed or untreated, and WHO indicates "the enforcement of countermeasures for the countries where access to diagnosis and treatment is not yet maintained is demanded".
Following these situations, the company expects that dissemination and penetration of TB - LAMP contribute greatly to solve these problems.
In addition to tuberculosis and other diseases listed above, EIKEN CHEMICAL and FIND also conduct collaborative research of reagents for leishmaniasis and Chagas disease.
Also, EIKEN CHEMICAL completed the development of a testing system “Simprova” that uses a next-generation compact fully automated genetic testing device and multi-item testing chip using the LAMP and started selling it in April 2020.
Due to the supply issues at overseas manufacturing contractors for equipment, the company is currently in the process of changing over to a domestic manufacturing partner and the new sales promotions are temporarily suspended.
This equipment fully automates the process from specimen preprocessing (nucleic acid extraction and purification) to amplification and detection. By developing the unique protocol that exploits the LAMP’s characteristics, the operation time that used to take over 2 hours with a conventional high purity nucleic acid extraction and purification device and an amplification and detection device combined, is now shortened to less than an hour.
At first, the company plans to release the respiratory organ infections panel and then the acid-fast bacterium disease panel and respiratory viruses panel, and will gradually increase the number of test items.
It is anticipated that “Simprova” will accelerate the spread of the LAMP and establish its position as the global standard in a newly created market.
*Gene amplification technology
Since the number of genes found in a genetic test sample is extremely small, to detect genes, the targeted gene must be amplified first. Gene amplification technology, therefore, is crucially important for genetic testing.
*African trypanosomiasis
An endemic found in tropical Africa; African trypanosomiasis is a serious tropical disease transmitted to HUMAN mbH by a protozoa called Trypanosoma brucei. The disease is transmitted by a tsetse fly. Trypanosoma in HUMAN mbH blood sucked by a tsetse fly develops and propagates inside the HUMAN mbH body in 2 to 5 weeks, before turning itself into a terminal Trypanosoma-type, which becomes a source of next round of infection. The disease causes fever, headache, and vomiting, and the patient falls into constant sleep. Since the patient cannot take meals, he or she becomes thin and complain of generalized weakness and, in many cases, leads to a complication and dies.
*Leishmaniasis
Leishmaniasis is a disease transmitted by a protozoon called leishmania, and has various types such as visceral leishmaniasis (also known as black fever), Brazilian leishmaniasis that affects skin and mucous membranes, and tropical leishmaniasis which affects skin. All these types are transmitted by blood-sucking insects, especially sandflies. Visceral leishmaniasis, after about three months incubation period, causes fever, sweating, diarrhea, etc. and, in about one month, causes a swollen liver and spleen, the patient develops an anemia and becomes weak if untreated, and may die in half a year to two years.
*Chagas disease
Found in southern U.S. as well as Central and South America, Chagas disease is an infectious disease transmitted by Reduviidae, a kind of blood-sucking Triatominae. The disease does not develop symptoms immediately after infection; it usually has a latency period of about 30 years. It causes symptoms such as inflammation of sinews, liver and spleen, myalgia, myocarditis, cardiomegalia encephalomyelitis, cardiac disturbance.
1-7 Return of earnings to shareholders
Positioning the return of earnings to shareholders as one of the most important management objectives, the company’s basic stance is to implement a stable dividend policy in consideration of strengthening the financial position and enriching internal reserves necessary for active business development. It aims to achieve a consolidated dividend payout ratio of 30% or higher.
The dividend payout ratio for FY 2022 is expected to be 30.3% while 35.8% is projected for FY 2023.
2. Fiscal Year Ended March 2022 Earnings Results
(1) Overview of consolidated results
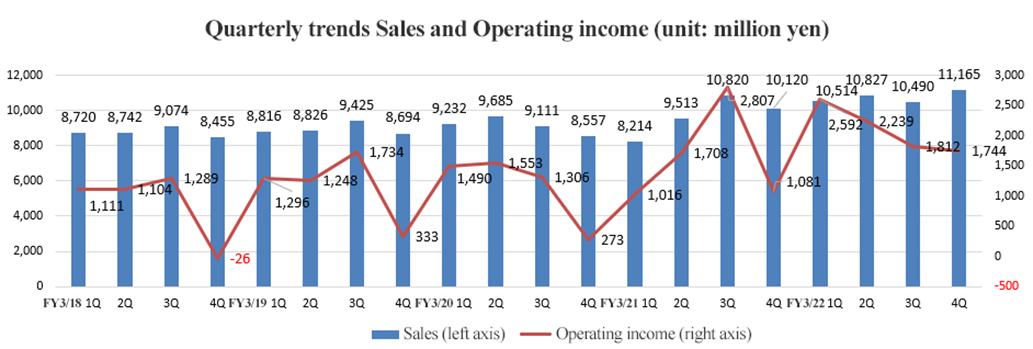
| FY 3/21 | Share | FY 3/22 | Share | YoY | Compared with forecast |
Sales | 38,667 | 100.0% | 42,996 | 100.0% | +11.2% | +6.4% |
Domestic | 31,772 | 82.2% | 34,128 | 79.4% | +7.4% | +9.0% |
Overseas | 6,895 | 17.8% | 8,868 | 20.6% | +28.6% | -2.4% |
Gross margin | 18,526 | 47.9% | 20,572 | 47.8% | +11.0% | - |
SG&A | 11,914 | 30.8% | 12,184 | 28.3% | +2.3% | - |
Operating income | 6,612 | 17.1% | 8,387 | 19.5% | +26.8% | +31.7% |
Ordinary income | 6,808 | 17.6% | 8,508 | 19.8% | +25.0% | +32.3% |
Net income | 5,044 | 13.0% | 6,218 | 14.5% | +23.3% | +26.7% |
*Unit: million yen.
Double digit growth of sales and profits. Exceeded the forecast.
In the fiscal year (FY) 2022, sales grew 11.2% year on year to 42.9 billion yen. Demand for detection reagents increased due to the resumption of various screenings/screening programs, the recovery trend in the number of outpatients, and the spread of COVID-19 in the fourth quarter. Overseas sales increased significantly mainly for fecal immunochemical test reagents.
Operating income increased 26.8% year on year to 8.3 billion yen. Gross profit, which was mainly from COVID-19 detection reagents, increased 11.0% year on year. On the other hand, sales expenses increased slightly as sales activities had to be restrained due to COVID-19.
With the rebound in infection rate in Japan after July, demand for COVID-19 detection reagents exceeded expectation while expenses were lower than expected, so both sales and profit exceeded the initial forecasts.
In response to the favorable business performance, the year-end dividend was increased by 10 yen/share to 31 yen/share, and the dividend for FY 2022 was 51 yen/share, up 10 yen/share from the previous fiscal year. The payout ratio was 30.3%.
(2) Sales by product
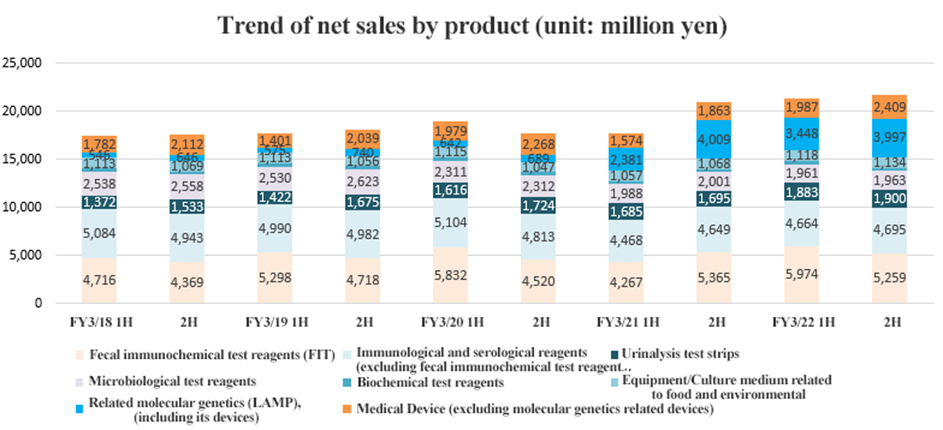
Products | FY 3/21 | FY 3/22 | YoY |
Fecal immunochemical test reagents (FIT) | 9,632 | 11,233 | +16.6% |
Immunological and serological reagents (excluding fecal immunochemical test reagents) | 9,117 | 9,359 | +2.7% |
Urinalysis test strips | 3,380 | 3,783 | +11.9% |
Microbiological test reagents | 3,989 | 3,924 | -1.6% |
Biochemical test reagents | 594 | 599 | +0.8% |
Equipment/Culture medium related to food and environmental | 2,125 | 2,252 | +6.0% |
Related molecular genetics (LAMP), (including its devices) | 6,390 | 7,445 | +16.5% |
Medical Devices (excluding molecular genetics related devices) | 3,437 | 4,396 | +27.9% |
Total sales | 38,667 | 42,996 | +11.2% |
*Unit: million yen.
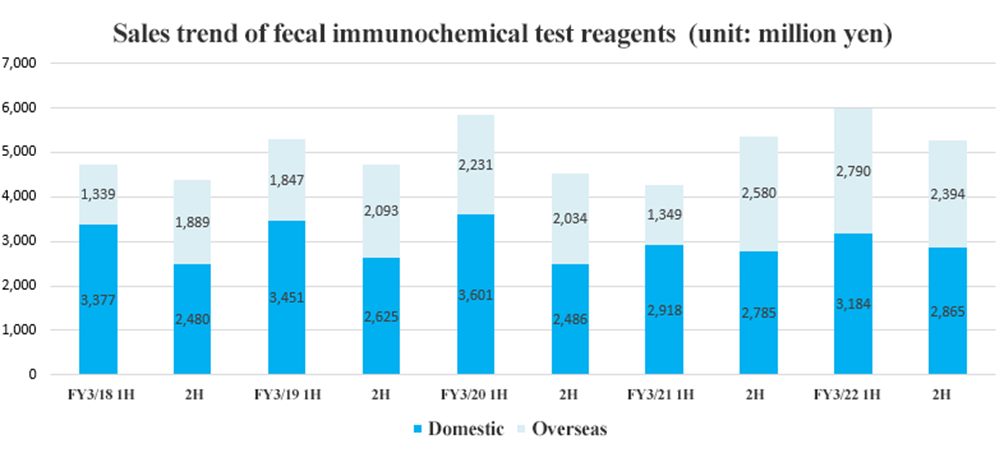
○Fecal immunochemical test reagents (FIT)
Sales in Japan increased 6.1% year on year while overseas sales increased 31.9% year on year. Screening programs that had been suspended due to the COVID-19 have been resumed in various countries. In particular, overseas growth was remarkable after stagnating over the same period in the previous fiscal year.
The second half, however, saw sales decreasing both in Japan and overseas from the first half.
| FY 3/21 | FY 3/22 | YoY |
Domestic | 5,703 | 6,049 | +6.1% |
Overseas | 3,929 | 5,184 | +31.9% |
Total | 9,632 | 11,233 | +16.6% |
*Unit: million yen.
○Immunological and serological reagents (excluding fecal immunochemical test reagents) microbiological test reagents
The number of outpatients is on a recovery trend and sales grew.
○Urinalysis test strips
Sales to Sysmex corporation increased due to both the medical checkup market and the number of outpatients being on a recovery trend.
○Microbiological testing reagents
Sales decreased due to the impact of COVID-19 tests and a decline in the number of tests for other infectious diseases.
○Related molecular genetics (LAMP)
The sales of novel COVID-19 detection reagents and genetic testing devices increased significantly.
The application of TB-LAMP and implementation of action programs for eliminating tuberculosis are continuing in various countries.
The company intends to contribute to the prevention of the spread of COVID-19 by providing a stable supply of testing reagents using the LAMP method and expanding this rollout globally.
 |
 |
 |
License/patent fee income declined 120 million-yen year on year to 1,165 million yen.
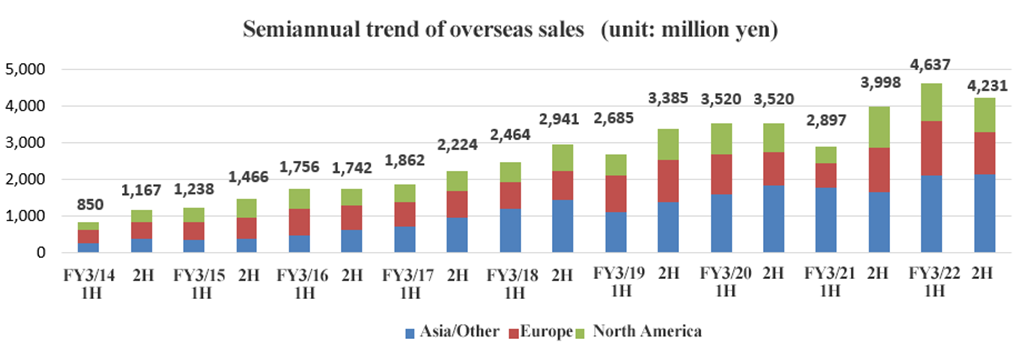
(3) Overseas trends
| FY 3/21 | FY 3/22 | YoY |
Overseas sales | 6,895 | 8,868 | +28.6% |
North America | 1,598 | 1,983 | +24.1% |
Europe | 1,853 | 2,611 | +40.9% |
Asia, others | 3,443 | 4,272 | +24.1% |
For OC | 3,929 | 5,184 | +31.9% |
Others | 2,966 | 3,684 | +24.2% |
*Unit: million yen.
The sales of fecal immunochemical test reagents increased significantly as medical examination programs in each country restarted.
In addition to the increased demand for FIT (fecal immunochemical test) due to changes in the screening scheme during COVID-19 pandemic, efforts were increased to tap into demand against the backdrop of the establishment of medical examinations through the internet and postal mail as well as triage of endoscopies.
Sales of urinalysis test strips and analyzers for Sysmex continued to be strong.
On a half-year basis, sales in Europe and North America decreased from the first half.
(4) Capital investment, R&D, Depreciation
| FY 3/20 | FY 3/21 | FY 3/22 | FY 3/23 Est. |
R&D | 3,333 | 3,086 | 3,408 | 3,400 |
Capital investment | 2,985 | 2,876 | 4,347 | 5,900 |
Depreciation | 1,629 | 1,711 | 2,058 | 2,080 |
* Unit: million yen.
R&D expenses were mainly costs associated with developing succeeding products to fecal occult blood tests and urine analysis devices. Capital investments were mainly in core system development and construction of new research building
(5) Financial status
◎Major BS
| End of March, 2021 | End of March, 2022 | Increase/Decrease |
| End of March, 2021 | End of March, 2022 | Increase/Decrease |
Current assets | 29,983 | 37,039 | +7,055 | Current liabilities | 12,772 | 12,533 | -239 |
Cash and deposits | 9,150 | 16,121 | +6,971 | Notes and accounts payable trade | 6,680 | 7,456 | +776 |
Notes and accounts receivable-trade | 12,298 | 11,956 | -342 | Income tax payable | 1,373 | 1,305 | -68 |
Inventory | 7,765 | 8,230 | +465 | Noncurrent liabilities | 1,239 | 4,175 | +2,935 |
Noncurrent assets | 25,701 | 25,473 | -228 | Total liabilities | - | 3,000 | +3,000 |
Property, plant and equipment | 12,768 | 15,275 | +2,506 | Net assets | 14,012 | 16,708 | +2,696 |
Intangible assets | 1,450 | 1,350 | -100 | Shareholder equity | 41,672 | 45,803 | +4,131 |
Investment and other assets | 11,481 | 8,847 | -2,633 | Total liabilities and net assets | 29,166 | 33,162 | +3,996 |
Total assets | 55,685 | 62,512 | +6,827 | Equity ratio | 55,685 | 62,512 | +6,827 |
*Unit: million yen. Accounts payable includes Electronically recorded monetary claims
Total assets increased 6,827 million yen from the end of the previous fiscal year to 62,512 million yen due to an increase in cash and deposit, inventory and construction in progress.
Total liabilities increased 2,696 million yen to 16,708 million yen due to the issuance of corporate bond.
Net assets increased 4,131 million yen to 45,803 million yen due to a rise in retained earnings.
As a result, equity ratio declined 1.5% from the end of the previous fiscal year to 72.8%.
3. Fiscal Year Ending March 2023 Earnings Estimates
(1) Estimate of consolidated results
| FY 3/22 | Share | FY 3/23 (Est.) | Share | YoY |
Sales | 42,996 | 100.0% | 40,000 | 100.0% | -7.0% |
Domestic | 34,128 | 79.4% | 30,260 | 75.6% | -11.3% |
Overseas | 8,868 | 20.6% | 9,740 | 24.4% | +9.8% |
Operating income | 8,387 | 19.5% | 4,540 | 11.4% | -45.9% |
Ordinary income | 8,508 | 19.8% | 4,570 | 11.4% | -46.3% |
Net income | 6,218 | 14.5% | 3,610 | 9.0% | -42.0% |
*Unit: million yen
Sales and profits decreased
For FY 2023, sales are expected to decrease 7.0% year on year to 40 billion yen, and operating income is expected to decrease 45.9% year on year to 4,540 million yen.
Demand for genetic testing for COVID-19 infections (COVID-19 detection reagents) is expected to decrease significantly from the previous fiscal year. The company expects a decrease in profit due to increased expenses toward the investment in R&D and the development of the management base, in addition to a decrease in sales of highly profitable COVID-19 detection reagents and patent rights income under the LAMP method.
The dividend is expected to be 35 yen/share, down 16 yen/share from the previous fiscal year. The expected dividend payout ratio is 35.8%.
(3)R&D・Capital investment・Depreciation costs
| FY 3/20 | FY 3/21 | FY 3/22 | FY 3/23(Est.) |
R&D | 3,333 | 3,086 | 3,408 | 4,300 |
Capital investment | 2,985 | 2,876 | 4,347 | 4,060 |
Depreciation costs | 1,627 | 1,711 | 2,058 | 2,310 |
*Unit: million yen
R&D expenses were mainly for the ongoing development of succeeding products to follow on from fecal occult blood tests and urine analysis devices.
Capital investment is expected to increase significantly due to the start of construction of the new research building. Depreciation costs will also increase.
The new research building to be constructed at the Nogi Plant will be positioned as a facility to help realize growth strategies, including "establish a global brand for gastrointestinal cancer," "create fundamental technologies to realize a diagnostic system for the three major infectious diseases," "develop high-value-added products," and "establish production technology in pursuit of quality and cost.”
The company will also pursue the achievement of breakthroughs by concentrating new information and technologies, for example, by improving creativity through open innovation in cooperation with third parties and consolidating dispersed laboratories. It is to be “A Place for Achieving Dreams,” somewhere that makes dreams come true and enables the pursuit of infinite possibilities.
It is scheduled to start operation in October 2022.
4. “EIKEN ROAD MAP 2030” and New Medium-Term Management Plan
To respond to the unfolding changes in its business environment and operate form a perspective of sustainability management, the company revamped its existing management framework “EIKEN ROAD MAP 2019” and redefined it as “EIKEN ROAD MAP 2030” with 2030 as the target year for its fulfillment. In addition, the company formulated its first Medium-Term Management Plan to achieve its goals.
4-1 Review of the Previous Medium-Term Management Plan (FY 2020 – FY 2022)
Both net sales and operating income significantly exceeded the targets for FY 2022, the final fiscal year of the Medium-Term Management Plan. Sales increased for the 22nd consecutive year and operating income reached a record high.
Operating income margin was 19.5% (target: 13.7%) and ROE was 14.3% (target: 10%), both exceeding the targets.
Externally, the drop in health screening and outpatients visits due to COVID-19 were negative factors, but positive factors such as increase in demand for products related to COVID-19 testing, increase in royalty income from LAMP method, and expansion of online and postal health screening using fecal occult blood tests overseas contributed significantly.
Internal factors include the unused budget for SG&A expenses due to COVID-19.
The company has steadily promoted business growth and strengthening of its base under the following basic strategies: (1) Developing foundations to increase management efficiency, (2) Promoting global expansion, (3) Maintaining domestic sales and increasing market share, and (4) Strengthening R&D ability. Within each of these targets, the company also identifies the following as its future issues and believes making these changes is essential for further growth: (1) Advancement of DX and human resource system reform; (2) Improvement of rates of uptake of colorectal cancer screening; search for demand for online screening, screening by post and endoscopic triage; and spread and establishment of testing for tuberculosis and malaria; (3) Improvement of rate of uptake of health checkups and screening ; and establishment and cultivation of the market for cognitive-function screening; (4) Improvement of the efficiency and speed of R&D; strengthening of core technologies and production technologies; and development of next-generation colorectal cancer screening tests.
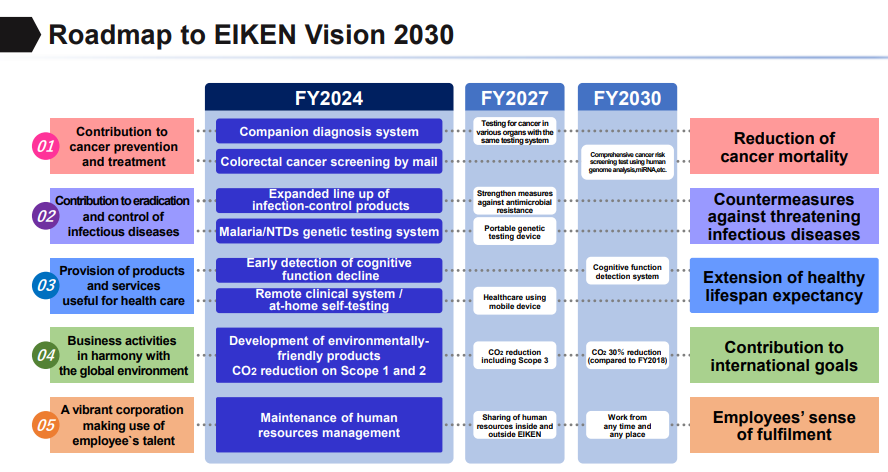
4-2 EIKEN ROAD MAP 2030
Based on the above recognition, the management concept has been redefined as EIKEN ROAD MAP 2030.
The EIKEN Vision 2030 clarifies the group’s vision for 2030 and sets forth Beyond the Field ~Team × Challenge~ as its slogan.
Each employee enhances their own abilities and expands the areas in which they can play an active role and creates new possibilities by bringing together the enhanced individual power beyond the boundaries and taking on challenges as a team. In addition, the company will take a step forward from its current business domain, innovate medical processes, and create the future of testing.
(From the reference material of the company)
(1) Business strategy: the priority business fields
While the current domain remains as its core business, the company has also set three focus business areas: ① Contribution to cancer prevention and treatment, ② Contribution to the eradication and control of infectious diseases, and ③ Provision of products and services useful for health care.
① Contribution to cancer prevention and treatment
The company has focused more on screening (prevention and early detection) so far, and especially for colorectal cancer it has built a global screening program, contributing to the reduction of mortality and the suppression of medical expenses through early detection.
On the other hand, selecting appropriate treatment is essential due to the significant medical expenses of cancer treatments. In addition to the prevention and early detection of cancer, to respond to these medical issues, the company aims to further reduce the mortality rate from cancer by developing and providing a testing system that covers the selection of therapeutic drugs and the determination of treatment effectivity.
② Contribution to the eradication and control of infectious diseases
As the countermeasures against threatening infectious diseases, the company will boost its product line-up and develop global genetic testing systems for tuberculosis, malaria, etc. In addition, by developing a simpler, faster, and more accurate infectious disease diagnosis system that can be used by anyone anywhere, the company wants to contribute to increasing access to medical care.
③ Provision of products and services useful for health care
To extend healthy lifespan expectancy, the company will expand remote clinical system and at-home self-testing areas and develop them into healthcare using mobile device. Ultimately, it aims to develop a monitoring system that can notify users of their health conditions around the clock even if they are not conscious of it.
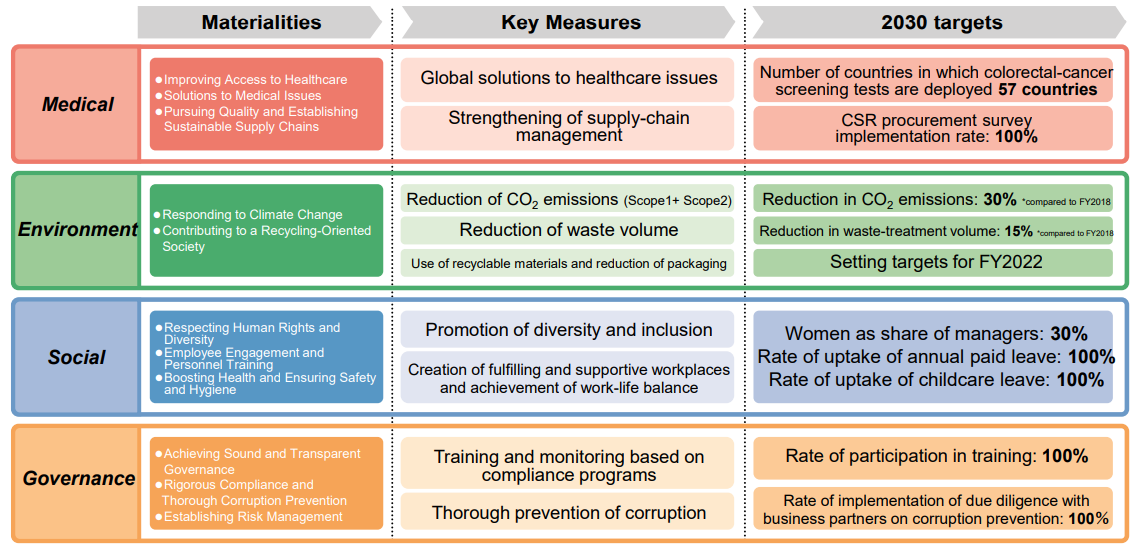
(2) Advancement of Sustainable Management
The company will promote sustainable management with setting their management strategies on business activities in harmony with the global environment and a vibrant corporation making use of employee’s talent.
The company believes being a vibrant corporation making use of employee’s talent will be the growth driver.
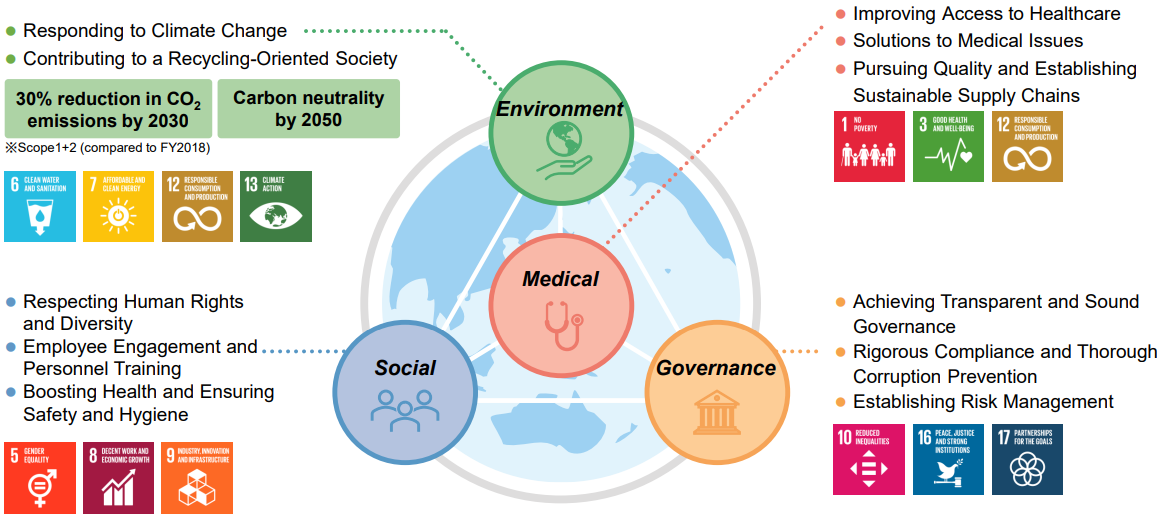
To realize a sustainable society, 11 materialities to be prioritized were identified, and specific action plans were put in place. Through resolving social issues, the company will aim to achieve the further enhancement of corporate value and the realization of a sustainable society.
(From the reference material of the company)
Materialities and KPI in detail: https://www.eiken.co.jp/en/sustainability/eiken/
(3) Objectives
① Financial targets
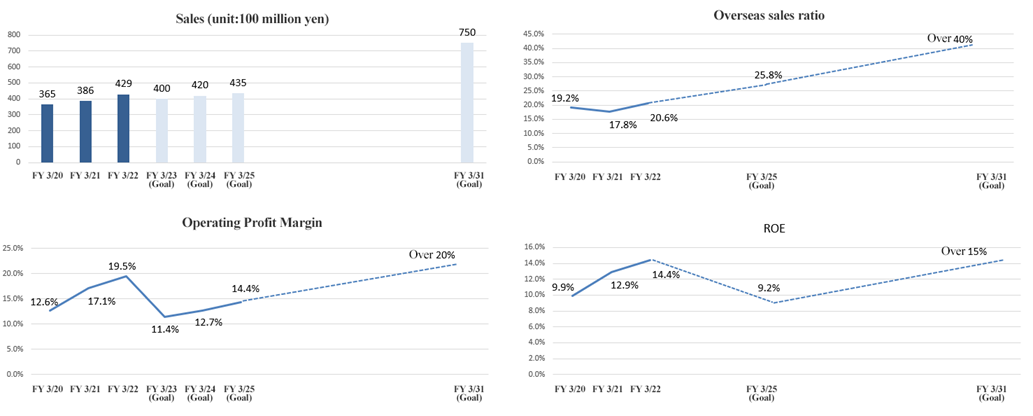
The targets for FY 2031 are as follows. The targets until FY 2025 are the figures according to the Medium-Term Management Plan (FY 2023 - FY 2025) described further down.
② Non-financial targets
As a company protecting the health of people worldwide, it is addressing issues not only of health care but also of the environment, society, and governance. For each materiality, it sets key performance indicators (KPIs) and monitors its progress toward achieving them. In addition to tracking its performance on the KPIs, the company reflects this performance in its evaluation of Executive Officers’ performance and in their remuneration.
Materialities and KPI in detail: https://www.eiken.co.jp/en/sustainability/eiken/
(From the reference material of the company)
4-3 Medium-term Management Plan (from FY 2023 to FY 2025)
This is the first Medium-Term Management Plan for EIKEN ROAD MAP 2030 and a three-year growth strategy.
The Plan outlines key measures in accordance with the vision of EIKEN ROAD MAP 2030 and responds to the currently accelerating paradigm shift in healthcare.
The company aims to advance the establishment management platform, promote personnel-focused management, enhance employee satisfaction and motivation, furnish an environment that fosters innovation and boost sustainable growth with steady improvement in profitability.
(1) Principal fields and key measures
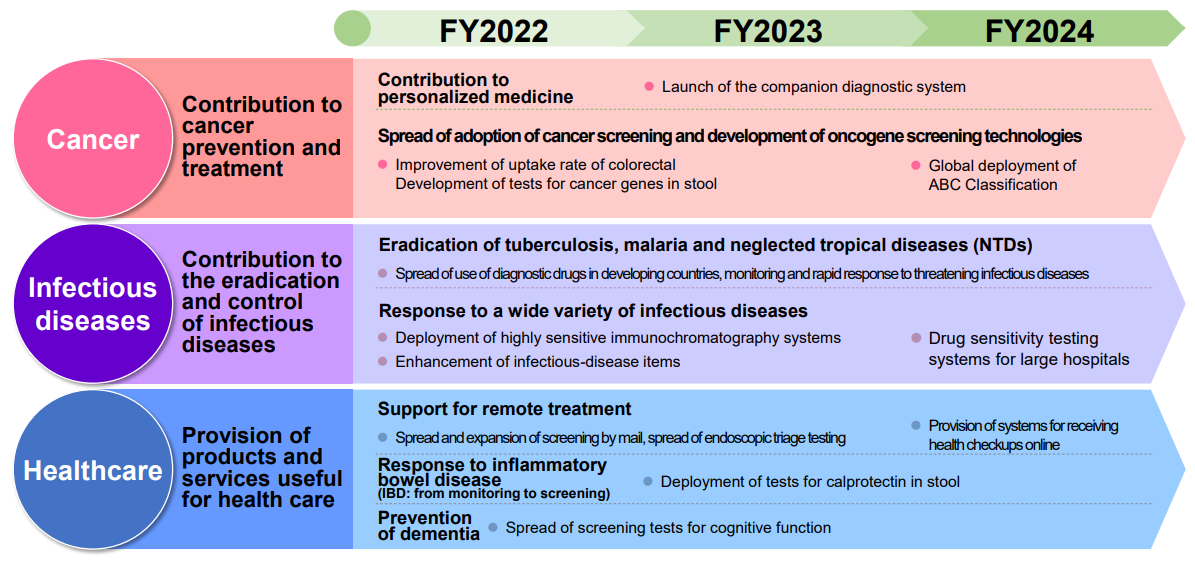
The key measures taken in the priority business areas of (i) contribution to cancer prevention and treatment, (ii) contribution to the eradication and control of infectious diseases, and (iii) provision of products and services useful for health care are as follows.
(From the reference material of the company)
In the field of colorectal cancer testing, to improve rates of uptake of colorectal cancer screening, the company will improve access to screening by expanding the number of screenings by mail and online screening. It will also develop a next generation fecal immunochemical test. The company aims to develop highly accurate medical laboratory technology to improve accuracy of colorectal cancer screening by increasing value-added of testing, for example, by cancer detection at earlier stages, and also, to narrow dawn the endoscopy target person and to develop tests to reduce patient’s physical burden.
The company will also focus on the development of a comprehensive genetic mutation testing system that detect multiple cancer gene mutations at once with next-generation sequencer (NGS). This system requires a shorter time to report results, is highly sensitive, and does not require many specimens. This system is expected to add target genes supporting new molecular targeted drugs and expand applications for many other cancers, help decide direction of cancer treatment (selection of molecularly targeted drugs) by blood tests.
(2) Establishing a management platform for sustainable growth
The company will further strengthen its management platform through the following five initiatives.
① Human resources strategy
The company is shifting to a wage system that focuses on job responsibilities and expertise and an evaluation system that brings out teamwork and challenges employees to pursue employee satisfaction and meaningful work.
② Structural reform
Based on the common understanding that our business field is the global market, the company is optimizing business processes and overhauling its systems with a view to speeding up decision-making.
③ IT strategy
The company is actively introduce and utilize AI and robots to promote DX in a wide range of business processes, including products and services, research and development, and manufacturing, to cultivate DX experts, and internal dissemination of DX.
④ Financial and capital strategies
By setting a target for cash conversion cycle, improving funding efficiency for business investment and diversifying its fundraising, the company will make agile and flexible investments to balance a sound financial base with operational expansion.
The company plans to spend a total of 28.4 billion yen in strategic investments over the next three years, including R&D, DX, work style reform, and facilities and equipment.
For M&A, rather than set a specific figure, this matter is considered separately.
Pursuit of stability and continuity in recognition of the importance of the return of earnings to shareholders as a management issue. The target dividend payout ratio is 30% or higher.
⑤ Governance
The company is strengthening its measures on ESG, with the aim of ensuring sound management that improves long-term corporate value. In addition, the company is advancing proactive IR and PR with high transparency, for example through the publication of integrated reports.
5. Interview with President Notomi
We interviewed President Notomi about EIKEN ROAD MAP 2030 and the new mid-term management plan.
Q:This time, you revised the EIKEN ROAD MAP 2019 management vision, newly redefined it as EIKEN ROAD MAP 2030 with 2030 as the goal and formulated the initial mid-term management plan for reaching the goal.
Why did you think that the redefinition was necessary?
Considerable changes occurred to the environment surrounding our company due to the spread of the COVID-19.
Regarding the genetic testing for the COVID-19, the structural issue of not being able to largely increase the number of tests compared to other countries became clear.
Moreover, as various tests and examinations are being put on hold at hospitals from the viewpoint of preventing infection, the need for testing and diagnostical methods that can be used remotely through post or the Internet increased further. Significant attention has also been brought to the necessity for online diagnosis regarding the medical system.
As our company also believed that providing COVID-19 detection reagents holds a significant social meaning, we put the highest priority on it and have responded to the demand. On the other hand, we have been impacted by the decrease in the number of patients at outpatient clinics and refraining from undergoing medical examinations in other categories.
Like this, seeing how considerably we were impacted by the COVID-19 both directly and indirectly and how significantly the times are changing amid the increasing importance of sustainability management, etc., we believed that it would be necessary to revise the current plan for once, redefined it through successive discussions, and conducted this announcement.
Q: EIKEN ROAD MAP 2030 states that you are going to promote business strategies focusing on the business fields of (i) contribution to cancer prevention and treatment, (ii) contribution to the eradication and control of infectious diseases, and (iii) provision of products and services useful for health care.
While you have so far held up the themes, developing foundations to increase management efficiency, promoting global expansion, maintaining domestic sales and increasing market share, and improving research and development ability, we get the impression that you are focusing on business fields which go a step beyond. What are your thoughts on this point?
This time we approached the business strategy in a different way. It was based on challenges which each in-house department should tackle so far, but we placed importance on the collaboration between different departments this time.
Our theme is to break down the barriers between departments so that they will cooperate with each other in taking on new challenges, which would also lead to the reform of our corporate culture.
This means research, production, and sales departments working together to resolve challenges left undone from the previous mid-term management plan one by one and promote the commercialization in the three fields.
Q:Please give us a brief comment on the three priority business fields of cancer, infectious diseases, and healthcare.
We assume that personalized medical care for cancer is the most important when it comes to developing the future pillar of business.
To elevate the effectiveness of the medicines and treatment methods in personalized medical care and use them more safely, there is a need to rightly determine whether it is appropriate to use a medicine for respective patients. This means checking the presence of specified genes, but for the sake of treatment rather than research, simpler and speedier testing is required.
The comprehensive genetic mutation testing system which our company is developing is a testing system encompassing everything from the selection of a medicine to the judgment of treatment effects in one test and we presume that it can contribute to the decrease in the mortality rate of cancer and reduction of medical expenses more than ever before.
The system itself is already complete and we are aiming to launch it on the market in FY 2023.
Regarding infectious diseases, we shall expand the product lineup and develop a global genetic testing system for tuberculosis, malaria, etc. This also implies a contribution to global health based on the recognition of our accomplishments concerning tuberculosis, so we are planning to make a certain and steady progress for each and every infectious disease.
Furthermore, we shall contribute to the improvement of access to medical care by developing a swift and accurate system for the diagnosis of infectious diseases, which will be simpler and available to anyone anywhere.
When it comes to health care, we shall broaden the range of remote examinations and home testing regarding fecal immunochemical tests. We are thinking of building a platform for this and going on to expand it to include other types of tests as well.
With regard to the prevention of dementia, we will first promote the platform to be adopted for checking whether dementia is suspected or not through a test for gauging the level of cognitive function instead of a diagnostic drug in general medical examinations and municipal medical checkups of residents.
Q:You have stated the goal of an overseas sales ratio of over 40% in FY 2031. What are the measures and challenges for achieving it?
The key is the expansion of sales of fecal immunochemical test reagents. Currently we focus on developed countries such as the U.S. and countries in Europe, but as there are many untapped markets, we shall open them up based on our company’s trust and advantage supported by extensive data.
In China, our testing method has been published in the guidelines for colorectal cancer screening tests. Currently there is an impact of Chinese government’s political measures in response to the COVID-19. Furthermore, as there is an incomparable number of tuberculosis patients, the genetic testing for tuberculosis is on a rapid rise. We have expectations for future development.
We have operated only in Shanghai, China and Amsterdam, the Netherlands until now, but we are also considering the establishment of operation bases in other areas.
Q:Next, please tell us on what kind of point you are going to focus in regard to the management of human resources.
The enrichment of education and training is one aspect, but we believe that it is important to elevate motivation in the whole company and we shall engage in the reform of our corporate culture with “challenge” being a keyword.
We shall create a system for taking on challenges and giving an opportunity for another challenge and evaluating the challenge itself even in case of a failure.
Moreover, “challenge” does not mean only taking on a challenge in new research and business, but also includes everyone supporting new initiatives for something that has not been done until now instead of confronting it with a negative opinion at all workplaces.
Q: Thank you very much. Lastly, please give a message to your shareholders and investors.
Sales and profit have significantly increased in these two years due to the COVID-19. As it is difficult to clearly estimate how this disease will spread in future and this fiscal year also saw the recoil from the rapid growth, so we are projecting a decrease in sales and profit, but we are successful in the steady cultivation of parts other than the COVID-19 which will become our foundations.
We shall continue the certain implementation of measures in the new Mid-Term Management Plan to aim for EIKEN ROAD MAP 2030 and the goal thereof and continue growing further, so please look forward to it.
6. Conclusions
The company predicts a decrease in both sales and profit in the current fiscal year. The demand for COVID-19 detection reagents, which had been increasing significantly in the previous two fiscal years, will be unavoidably on a reactionary decline from this fiscal year onward due to the subsiding infection rate. However, when comparing the CAGR (Compound Annual Growth Rate) for the five years before COVID-19 pandemic and the five years until the final fiscal year of the post-pandemic Medium-Term Management Plan, there is no significant difference between the two of them, as both are just over 3%. On the other hand, as COVID-19 pandemic subsides, the number of conventional tests and medical inspections is on a recovery trend, and the growth of fecal immunochemical test reagents overseas in particular has been remarkable.
Although the stock price has been on a downward trend since the beginning of this fiscal year in response to the forecasts for the current fiscal year, it should be noted that there has been no significant change in the speed of the company's growth, and it is also planning to accelerate the growth speed thereafter. We look forward to witnessing how the company will progress through the various measures to realize its visions set out in EIKEN ROAD MAP 2030.
<Reference: Regarding Corporate Governance>
◎ Organization type, and the composition of directors and auditors
Organization Type | Company with a nominating committee and others |
Directors | 8, including 5 outside ones (Including 1 female) |
Nominating Committee | 3, including 2 outside ones |
Compensation Committee | 3, including 2 outside ones |
Audit Committee | 4, including 3 outside ones (Including 1 female) |
◎ Corporate Governance Report
Last updated: submitted on June 22, 2022
<Basic Policy>
Our policy for corporate governance is based on our management philosophy, management vision, and motto.
*Management philosophy
We protect the health of people through healthcare services.
*Management vision
In order to protect the health of people, EIKEN Group offers reliable products and services as a pioneer in checkups, to improve its corporate value.
*Motto
“EIKEN” winning trust with quality and growing with technology
To improve our corporate value by enhancing the soundness, speed, and transparency of our business administration, we are enriching our corporate governance while emphasizing the viewpoint of shareholders and recognizing it as an important managerial mission.
Our company has adopted a corporate structure that has a nominating committee, separating the business execution function and the supervisory function of the management. Important items regarding the basic policy for business administration are determined through the deliberation of the board of directors, and business execution is conducted swiftly and smoothly under the appropriate chain of command, in accordance with our in-company regulations and rules.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)>
The company has implemented every principle detailed in the Corporate Governance Code.
Disclosure Based on Each Principle of the Corporate Governance Code (Excerpts)
Principles | Disclosure content |
[Principle 1-3 Objective of Capital Policy] | Our company's basic capital policy is to improve capital efficiency and provide sustainable and stable shareholder returns to maintain and increase shareholder value. With respect to shareholder returns, we aim to maintain a consolidated dividend payout ratio of 30% or higher, taking into consideration the enrichment of internal reserves necessary for strengthening our financial position and aggressive business operation. When implementing capital policies (including capital increase, MBO, etc.) that would result in a change in control or significant dilution, the Board of Directors will fully discuss the necessity and rationality of the policy and ensure that appropriate procedures are followed. In addition, we will make efforts to provide sufficient explanations to shareholders and investors. |
[Principle 1-4 Strategically Held Shares] | 1. Policies on Strategic Holding of Listed Stocks Our basic policy is to hold shares of business partners only when we deem it reasonable to do so for the smooth promotion of business activities, maintenance of business relationships, business affairs, and capital alliances, and to continue to strategically hold these shares as long as we judge that they will contribute to the development of our business. To assess the value of holding these shares, the Board of Directors discusses annually whether the return (based on quantitative factors such as dividends and trading conditions, as well as a comprehensive assessment of importance in terms of management strategy and business relationships) is commensurate with the risk, in light of the cost of capital. We will sell off stocks that are deemed to have little benefit in holding, taking into consideration stock price trends and other factors. As for listed stocks, the Board of Directors discussed the issue at its meeting on April 28, 2021, and decided to continue holding the stocks of five companies in the fiscal year 2021. 2. Standards for Exercising Voting Rights for Strategically Held Stocks Our company exercises the voting rights for strategically held shares based on a comprehensive judgment of factors such as the state of corporate governance of the company concerned, whether the proposal contributes to improving shareholder value, and the impact on our company. |
[Supplementary Principle 2-4 (i) To Ensure Diversity in the Appointment of Core Personnel, etc.] | Our company actively and continuously hires and appoints personnel without regard to nationality, gender, or time of employment. As of April 1, 2022, there are 19 female managers, accounting for 14.8% of the total number of managers. In addition, 37 mid-career hires accounted for 28.9% of the total number of managers. Moving forward, from the perspective of the further promotion of diversity, we aim to increase the percentage of female managers to 30% by 2030. Under this policy, our company has formulated the Vision for Ability Development for ideal human resources, and are promoting the activities of a diverse range of human resources regardless of nationality, gender, or age. In addition, we have adopted various employment systems, including a telework system and a flextime system with no core working hours, to increase the diversity of work styles and create an environment in which employees can exert their abilities to the maximum degree. |
[Supplemental Principle 3-1(iii) Sustainability Initiatives, etc.] | Our company has been striving to solve various social issues through business activities based on our management philosophy of "Protect the health of the public through health care service." To proactively promote sustainability throughout our corporate group, we have formulated Sustainability Policies and established a Sustainability Committee, chaired by the Representative Director and President and composed of executive officers managing each function and business group to promote activities. The contents of the Sustainability Committee are reported and supervised by the Board of Directors. Under the EIKEN ROAD MAP 2030, we have identified material issues for the realization of a sustainable society, reflected them in specific action plans, and are promoting initiatives while monitoring progress by setting indicators (KPIs).Our sustainability concept, policy, promotion system, and initiatives are disclosed on our website.https://www.eiken.co.jp/en/sustainability/ In addition, recognizing the risks that climate change poses to financial markets, we are further promoting our existing initiatives on climate change and disclosing information based on the TCFD recommendations. Moving forward, we will continue to conduct analysis and discussion, and gradually expand our information disclosure. Details are available on our websitehttps://www.eiken.co.jp/en/sustainability/environment/weather/ Regarding investment in human capital, our company will promote management focused on human capital to enhance employee satisfaction and job satisfaction to create an environment that triggers innovation, and achieve sustainable growth and steady improvement in profitability. Details are available on our website.(https://www.eiken.co.jp/en/sustainability/environment/weather/) Regarding investment in intellectual property, we will steadily grow our existing businesses and allocate management resources to applying our core technologies to peripheral businesses and developing new businesses through open innovation with external parties. Details are available on our website.https://www.eiken.co.jp/en/rd/ |
[Principle 5-1 Objective Regarding Constructive Dialogue with Shareholders] | Our company has established a Disclosure Policy approved by the Board of Directors, which discloses basic policies, information to be disclosed, information disclosure methods, the quiet period, etc. Our company has dialogues with shareholders to a reasonable extent to contribute to sustainable growth and medium/long-term improvement of corporate value. We have established a system in which the Sustainability Promotion Department manages IR, and the General Manager of the Corporate Administration Division who oversees the Sustainability Promotion Department, has been placed in the role of executive officer in charge of IR in an earnest effort to gain understanding and trust through dialogue with our shareholders and investors. The General Manager of the Corporate Administration Division concurrently oversees the Corporate Planning Department, the Accounting Department, the Human Resources & General Affairs Department, and other departments related to IR, and ensures close information sharing and collaboration among these departments. In terms of dialogue with shareholders, our company holds a briefing session for analysts and institutional investors twice a year at the time of the announcement of annual financial results and the financial results for the second quarter, at which the Representative Director and President provides explanations and has dialogues with shareholders. Individual meetings with shareholders and investors are handled by the Sustainability Promotion Department. Depending on the requests of shareholders and investors and the number of shares held, senior executives of the management team are available for interviews to a reasonable extent. Key opinions of shareholders and investors obtained through dialogue are regularly reported to the Board of Directors by the executive officer in charge of IR. Our company conducts dialogues with shareholders and investors in accordance with its Disclosure Policy, and in addition to taking sufficient care not to include insider information, our company has established internal rules in accordance with applicable laws and regulations, and manages information appropriately based on these rules. |
This report is not intended for soliciting or promoting investment activities or offering any advice on investment or the like, but for providing information only. The information included in this report was taken from sources considered reliable by our company. Our company will not guarantee the accuracy, integrity, or appropriateness of information or opinions in this report. Our company will not assume any responsibility for expenses, damages or the like arising out of the use of this report or information obtained from this report. All kinds of rights related to this report belong to Investment Bridge Co., Ltd. The contents, etc. of this report may be revised without notice. Please make an investment decision on your own judgment. Copyright(C) Investment Bridge Co., Ltd. All Rights Reserved. |
With “We aspire to solve all social issues together with investors and companies and create a bright and prosperous future” as its social mission (purpose) of the company, Investment Bridge Co., Ltd. provides various services, including the creation of Bridge Reports, as a bridge connecting investors and companies.
We advocate Supportive Investment™ in which investors and companies work together to create a bright and prosperous future.
The back issues of Bridge Report (EIKEN CHEMICAL : 4549) and the contents of Bridge Salon (IR seminar) can be found at: for more information www.bridge-salon.jp/


