Bridge Report:(4598)Delta-Fly Pharma the second quarter of Fiscal Year ending March 2021
 President Kiyoshi Eshima | Delta-Fly Pharma, Inc.(4598) |
 |
Corporate Information
Exchange | TSE Mothers |
Industry | Pharmaceutical products (manufacturing industry) |
President | Kiyoshi Eshima |
Address | 37-5 Nishikino, Miyajima, Kawauchi-cho, Tokushima-shi, Tokushima |
Year-end | End of March |
URL |
Stock Information
Share Price | Shares Outstanding | Total Market Cap | ROE(Actual) | Trading Unit | |
¥1,663 | 4,504,600 shares | ¥7,491million | -56.0% | 100 shares | |
DPS(Estimate) | Dividend Yield (Estimate) | EPS(Estimate) | PER(Estimate) | BPS(Actual) | PBR(Actual) |
¥0.00 | - | ¥-188.70 | - | ¥456.47 | 3.6 times |
*Share price is as of closing on November 24. DPS and EPS were taken from the brief financial report for the second quarter of the term ending March 2021. ROE and BPS were taken from the brief financial report for the term ended March 2020.
Earnings Trends
Fiscal Year | Net Sales | Operating Income | Ordinary Income | Net Income | EPS | DPS |
Mar. 2017 (Actual) | 902 | 328 | 323 | 305 | 88.31 | 0.00 |
Mar. 2018 (Actual) | 150 | -243 | -244 | -246 | -71.20 | 0.00 |
Mar. 2019 (Actual) | - | -592 | -671 | -673 | -170.16 | 0.00 |
Mar. 2020 (Actual) | 100 | -1,545 | -1,552 | -1,555 | -348.32 | 0.00 |
Mar. 2021 (Estimate) | 300 | -850 | -850 | -850 | -188.70 | 0.00 |
*Unit: million-yen, yen
*The estimated values were provided by the company. 500-for-1 share split was conducted on Jun. 25, 2018. EPS is adjusted retroactively.
This report introduces earnings trends, progress of the development etc. of Delta-Fly Pharma, Inc.
Table of Contents
Key Points
1. Company Overview
2. Earnings Trends
3. Growth Strategy
4. Conclusions
<Reference: Regarding Corporate Governance>
Key Points
- The company develops anticancer drugs under the original concept of “module drug development,” which means that they develop new anticancer drugs that have an improved balance between clinical efficacy and safety with fewer side effects, by using the existing active substances with anticancer property as “modules (components)” and designing dosage and administration, combination methods, etc. with ingenuity.
- “Module drug development” has benefits for patients, including the improvement of treatment effects, the reduction of side effects and costs, and also benefits for development, including the high exclusiveness in patenting, the swiftness of development, and low development risk. The company currently has 6 drug pipelines, and 4 candidate drugs are under clinical studies, and for the other 2 candidate drugs are being prepared for the clinical studies.
- In addition to module drug development, the company is characterized by the specialization in development of anticancer drugs, the development by experienced members, and efficient business operation utilizing external resources.
- In the second quarter of the term ending March 2021, the company recorded milestone revenue of 100 million yen related to the licensing contract with Nippon Chemiphar Co., Ltd.,. The number of Medical institutions undertaking the clinical studies for pipelines in development, and the number of patients’ enrollment were increased, and progress was made in the preparation for new clinical trials, but the R&D expense decreased by 175-million-yen, year on year. As a result, operating loss shrank 262-million-yen year on year to 463 million yen.
- There is no change in the full-year forecast for the fiscal year ending March 2021. As for the business revenue, the company expects to earn 300 million yen as milestone compensation for licensing contracts. The company expects to finish the clinical phase I test of DFP-14927 in the U.S. while further increasing the number of patients’ enrollment of DFP-10917 clinical phase III study in the U.S.
- Further, as the patients’ enrollment of the clinical phase II study of DFP-14323 in Japan was finalized, the company plans to make preparations, conducting the next clinical phase III study (large-scale controlled trial) jointly with Chinese pharmaceutical companies. Also, as for DFP-17729, for which the company has a partnership with Nippon Chemiphar Co., Ltd., it started domestic clinical studies and will proceed with the development of these drug pipelines. As the company had ordered the manufacturing of active pharmaceutical ingredients to a contract manufacturing company (CMO) and preparations in the previous term earlier than planned, it expects R&D expenses to decrease.
- The spread of the novel coronavirus seemed to have slowed down the patients’ enrollment for the clinical studies of DFP-10917 and DFP-14927 in the U.S., but there seems to be no major impact as of now. On the other hand, while the market scale is medium-sized, steady progress is being made in development and commercialization of DFP-14323, which is to be the third product including the Chinese market by fiscal 2025. DFP-14323 entered the preparation stage for its clinical phase III study. Progress is also being made in negotiations with their partner, and we have high expectations regarding the release of DFP-14323.
1. Company Overview
Delta-Fly Pharma upholds the corporate ethos: “To provide treatment methods recommendable for cancer patients and their families with peace of mind, by diagnosing all states of cancer patients rather than focusing on only cancer,” and develops anticancer drugs under the original concept of “module drug development,” which means that they develop new anticancer drugs that have an improved balance between clinical efficacy and safety with fewer side effects, by using the existing active substances with anticancer property as “modules (components)” and designing dosage and administration, combination methods, etc. with ingenuity.
1-1 Corporate history
The President Eshima, who was born in Tokushima Prefecture, graduated from Nagoya Institute of Technology, completed the master’s course of Tokyo Institute of Technology, and joined the Otsuka Group, a pharmaceutical company in Tokushima Prefecture, which is his hometown. Then, he was assigned to TAIHO Pharmaceutical Co., Ltd., which is a business company of the Otsuka Group.
Immediately after joining the company, he was dispatched to Faculty of Science and Engineering, Waseda University, and engaged in the development of pharmaceuticals, especially new medicines composed of functional polymers, as a researcher for about 12 years. When he was in the section that seeks seeds of pharmaceutical products in TAIHO Pharmaceutical, he saw how the business administration of U.S. bio ventures was carried out. That stirred his willingness to become independent, manage a pharmaceutical company by himself, and create medicines with a new approach, rather than engaging in development in the R&D section of a leading pharmaceutical company. He also aimed to develop a business while not only creating medicines, but also considering what he can do for patients in front of him. In 2010, when he was 61 years old, he resigned from TAIHO Pharmaceutical, and established Delta-Fly Pharma. The company is committed to the development of anticancer drugs with fewer side effects and friendly to patients through module drug development. As of September 2020, the company has 6 drug pipelines.
It was listed in Mothers of Tokyo Stock Exchange in October 2018.
1-2 Corporate ethos and management philosophy
The corporate name “Delta-Fly” is derived from a “dragonfly.” Since dragonflies only go forward, and do not go backward, they represent the unflagging spirit, and they are also called “winning insects.” Namely, the corporate name implies the firm resolve to develop pharmaceutical products.
Corporate ethos | To provide treatment methods recommendable for cancer patients and their families with peace of mind, by diagnosing all states of cancer patients rather than focusing on only cancer, |
As mentioned later, the company considers that its social mission is not to develop anticancer drugs only for eradicating “cancer,” but to provide anticancer treatment with reasonable price while curbing side effects, which are serious issues with anticancer drugs, so that patients and their family members can use it without worry.
1-3 Environment surrounding the company
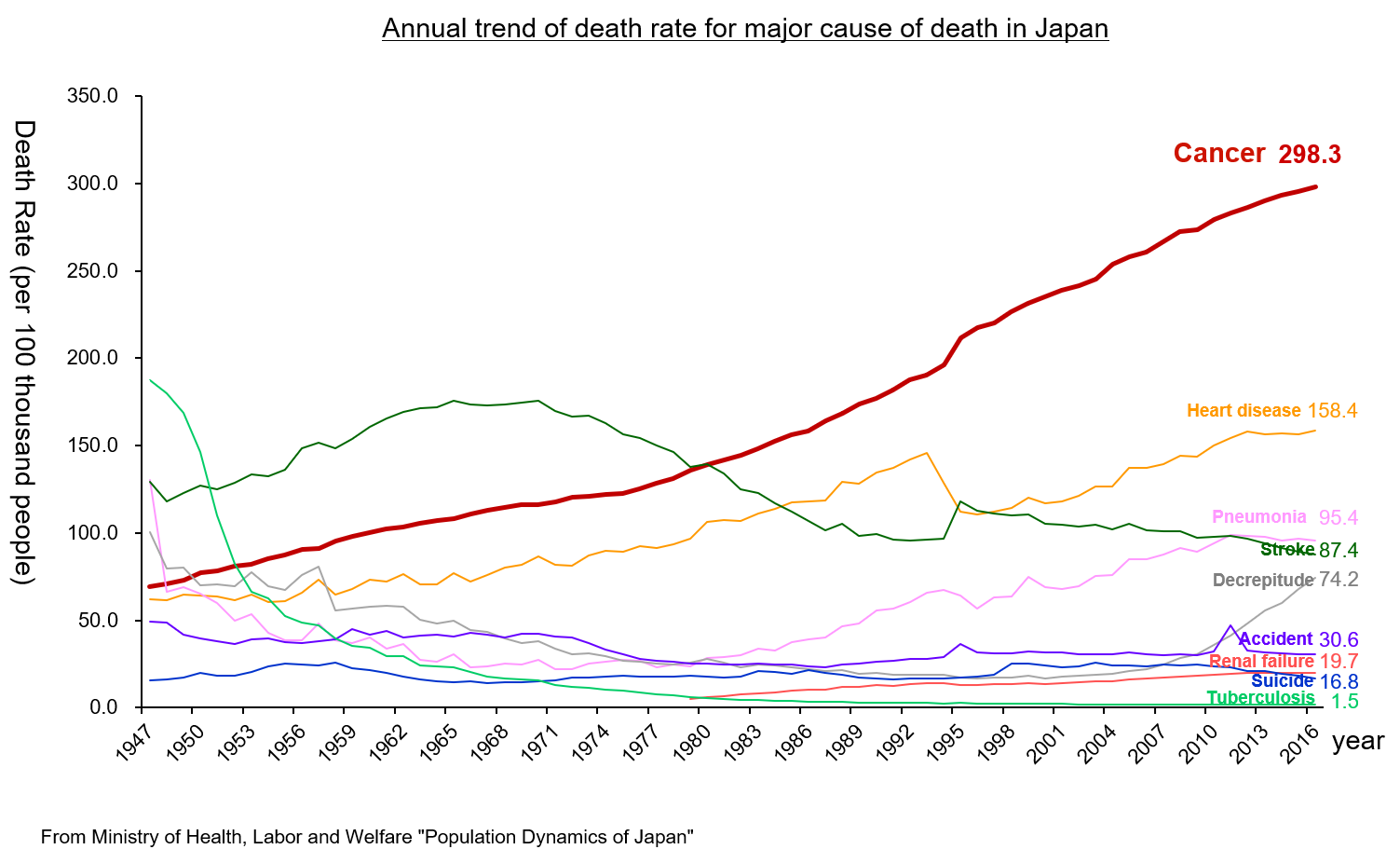
According to “the demographic statistics in Japan in 2018” published by the Ministry of Health, Labor and Welfare, the mortality rate (number of deaths per 100,000 people) of malignant neoplasm (cancer) was the highest: 298.3 in 2016. It has been the highest for over 30 years since it replaced cerebrovascular disease, whose mortality rate was 134.3 while that of malignant neoplasm was 142.0, in 1981. It is increasing year by year.
It is said that the incidence of cancer is growing due to the aging of the population, the change in lifestyles, including dietary habits, etc.
(Taken from the reference material of the company)
(Taken from the reference material of the company)
In these circumstances, various anticancer drugs are used, and new medicines are being developed. But, as publicly known, the side effects of anticancer treatment are significant, so there are considerable needs for the reduction of side effects from the viewpoint of improving the quality of life (QOL) of patients.
(Mechanism of side effects)
Since cancer cells rapidly divide and proliferate, anticancer drugs are designed to kill rapidly growing cancer cells. However, anticancer drugs affect not only cancer cells, but also the normal cells that rapidly divide, such as blood cells produced in bone marrow, the cells of digestive organs, the cells of genitals, and hair root cells, causing side effects, such as nausea, vomiting, hair loss, and fatigue.
1-4 Business contents
1-4-1 Delta-Fly Pharma’s method for creating medicines: Module drug development
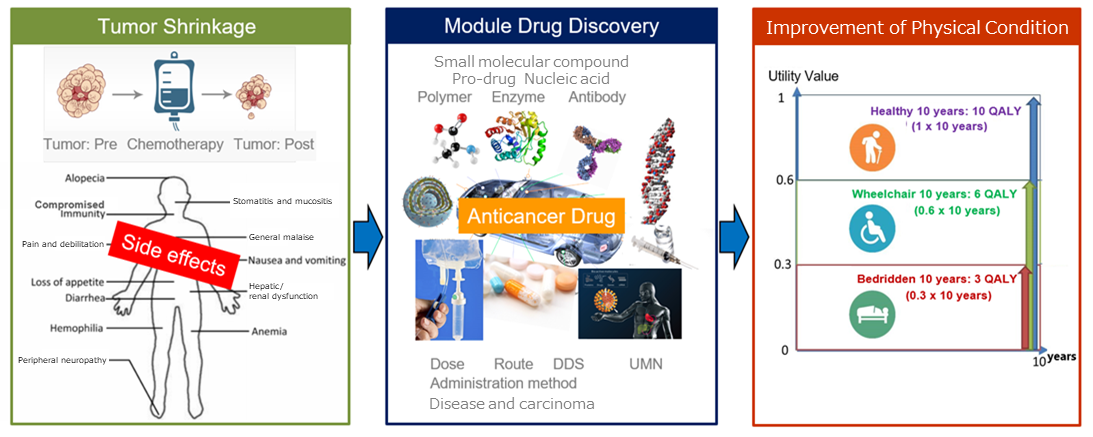
What distinguishes the company most among a lot of bio ventures is its concept for developing medicines: “module drug development.”
(Taken from the reference material of the company)
“Module drug development” means the development of new anticancer drugs that have an improved balance between clinical efficacy and safety by using the existing active substances with anticancer property as “modules (components)” and designing dosage and administration, combination methods, etc. with ingenuity.
Through “module drug development,” the company focuses on not only “cancer circumstances” but also the whole conditions of “cancer patients,” improves the anticancer drugs with fewer side effects and which have various side effects in a multifaceted manner, and produces medicines whose side effects are so fewer that you can recommend them to cancer patients and their families.
(Advantage of module drug development)
Merits for patients | ・Since medicines are created based on data on patients, treatment effects are expected to improve. ・Since medicines are created based on data on patients, conventional side effects are expected to reduce. ・The number of fundamental and clinical tests is small and their periods are short; accordingly, their costs are not considerable. |
Merits for development | ・Since medicines can be patented due to novelty and inventive steps, they will have high exclusivity. ・Since medicines are developed based on data on patients, development speed is high. ・Since medicines are developed based on data on patients, development risk is low. |
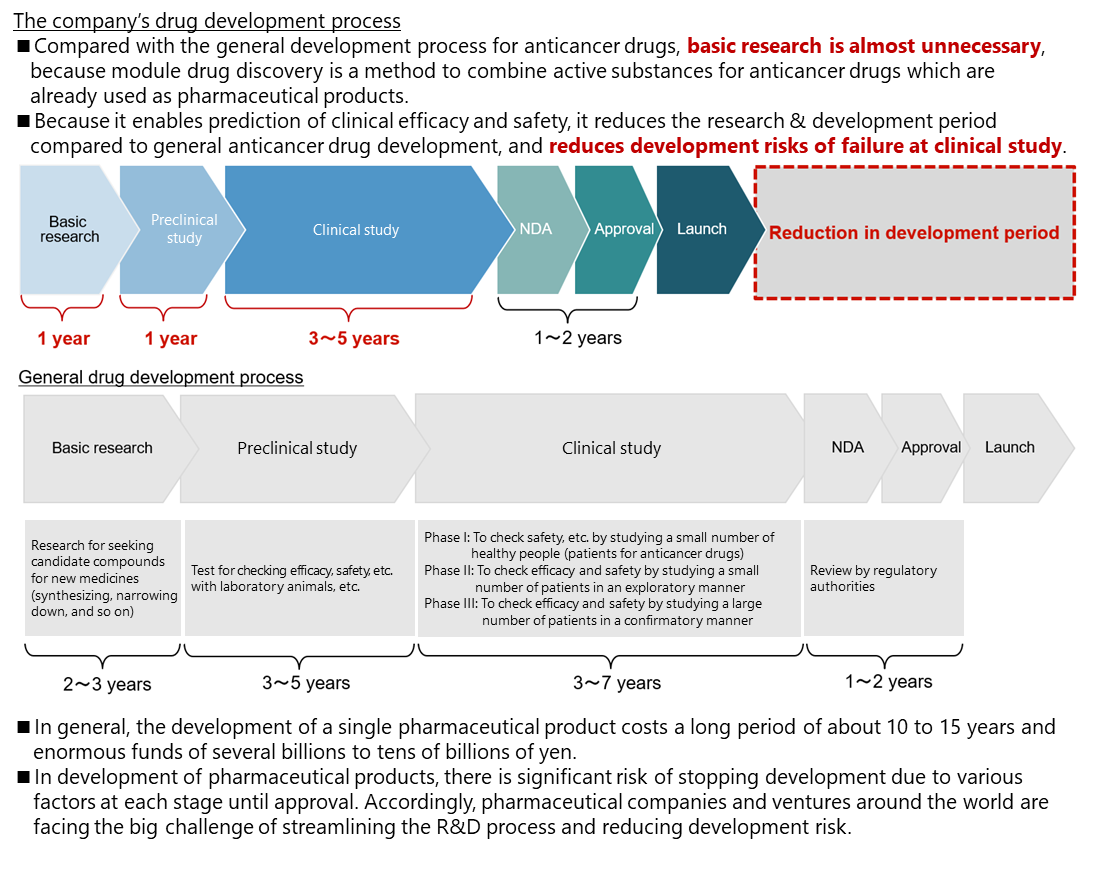
In general creation of anticancer drugs, chemicals that act on the cancer-specific parts are extracted at the stage of fundamental search and research, and possible chemicals become candidates for anticancer drugs. However, it is necessary to check their functions at the clinical stage and demonstrate efficacy and safety through clinical tests. Accordingly, the R&D period from the basic stage is long.
Meanwhile, “module drug development” does not require fundamental search or research so much, because the active substances of already used anticancer drugs are combined, and it is possible to predict efficacy and safety at the clinical stage. Accordingly, it is possible to start clinical tests in one to two years after the start of medicine development. Like this, compared with general development of anticancer drugs, the R&D is more efficient, the development period is shorter, and the risk of development, including the failure in clinical tests, is lower.
In addition, when focusing on the issues with the treatment of cancer patients, the combination of off-patent pharmaceutical products by utilizing the knowledge and know-how of anticancer drugs enables them to be patented as new anticancer drugs.
(Taken from the reference material of the company)
Nowadays, an increasing number of pharmaceutical companies engage in drug-repositioning activities to discover new effects of generic and existing medicines, for the purpose of reducing the cost for new drug development.
These are the same as “module drug development” in that existing medicines are used. It is difficult to patent these drugs based on generic medicines and drug repositioning because of the lack of novelty and inventiveness. On the other hand, “module drug development” will make all developed drugs patented. This is a defining difference.
As long as they try to solve the problems with anticancer drugs, they can create totally new anticancer drugs. Therefore, the company is certain that “module drug development” will bring significant innovation to methods for creating medicines.
1-4-2 Business and revenue models
(Business model: to develop an efficient R&D system)
Before a new pharmaceutical product is released, it is common that “fundamental research” is first conducted, “preclinical tests (tests for checking the pharmacological actions, in-vivo kinetics, harmful effects, etc. by using animals)” and “clinical tests (scientific tests for studying the effects of pharmaceutical products, treatment technologies, etc. on human bodies)” are carried out, applications are submitted to authorities to obtain approvals, products are manufactured, and then surveys are conducted after manufacturing, marketing, and sale.
In these processes, Delta-Fly Pharma concentrates on the management of R&D, while outsourcing meticulous tasks to excellent external R&D companies and manufacturers inside and outside Japan. The company has actualized an efficient R&D system in cooperation with external cooperative institutions according to development phases. It also engages in the R&D for new anticancer drugs by using a drug delivery system in collaboration with Sanyo Chemical Industries, Ltd. (1st section of TSE; 4471).
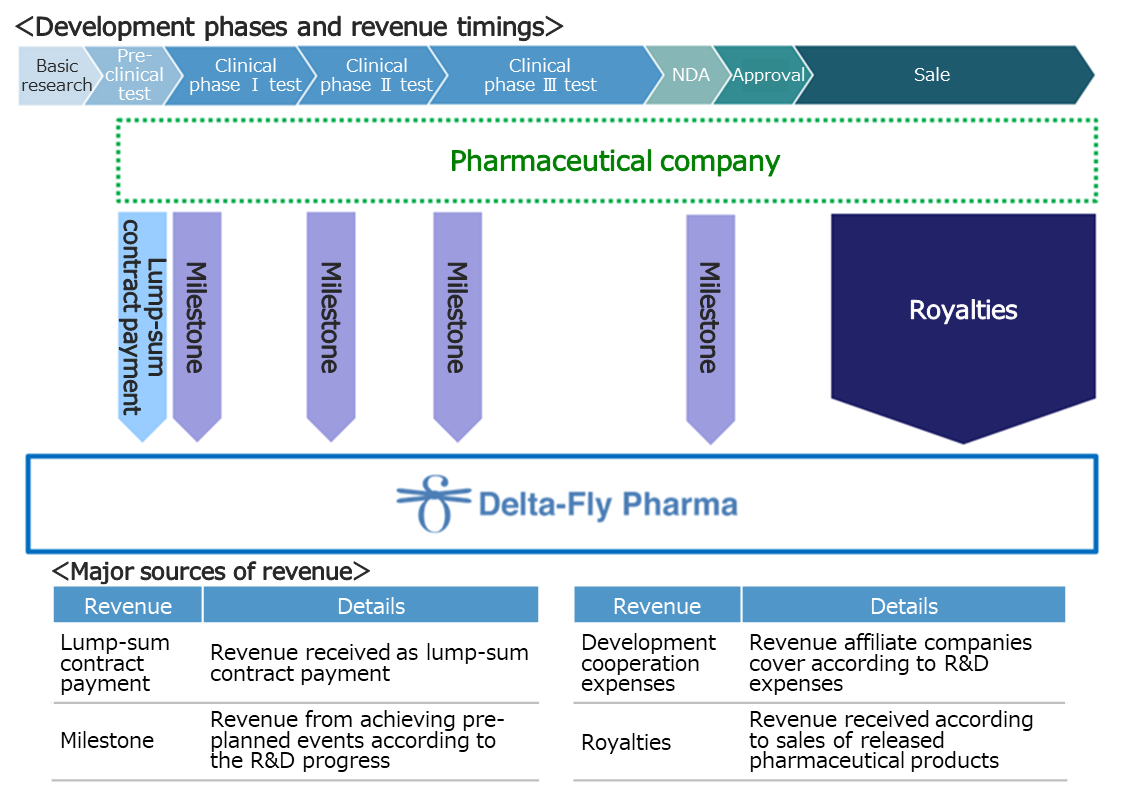
(Revenue model)
At the R&D stage, the main revenue sources are “lump-sum contract payment” for contracts with affiliated pharmaceutical companies, “milestone,” and “cooperation funds for development.” If collaborative products are released, the company will receive royalties according to sales.
Currently, Delta-Fly Pharma collaborates with the following two pharmaceutical companies.
Nippon Shinyaku Co., Ltd. (1st section of TSE, 4516) | Signed a contract for an exclusive license right for DFP-10917 in Japan. |
Nippon Chemiphar Co., Ltd. (1st section of TSE, 4539) | Signed a contract for an exclusive license right for DFP-17729 in Japan. |
* The company signed a contract for an exclusive license right with Kyowa Chemical Industry Co., Ltd. (unlisted) for DFP-14323, in Japan, but consented to cancel the contract in November 2020.
(Taken from the reference material of the company)
1-4-3 Drug pipelines
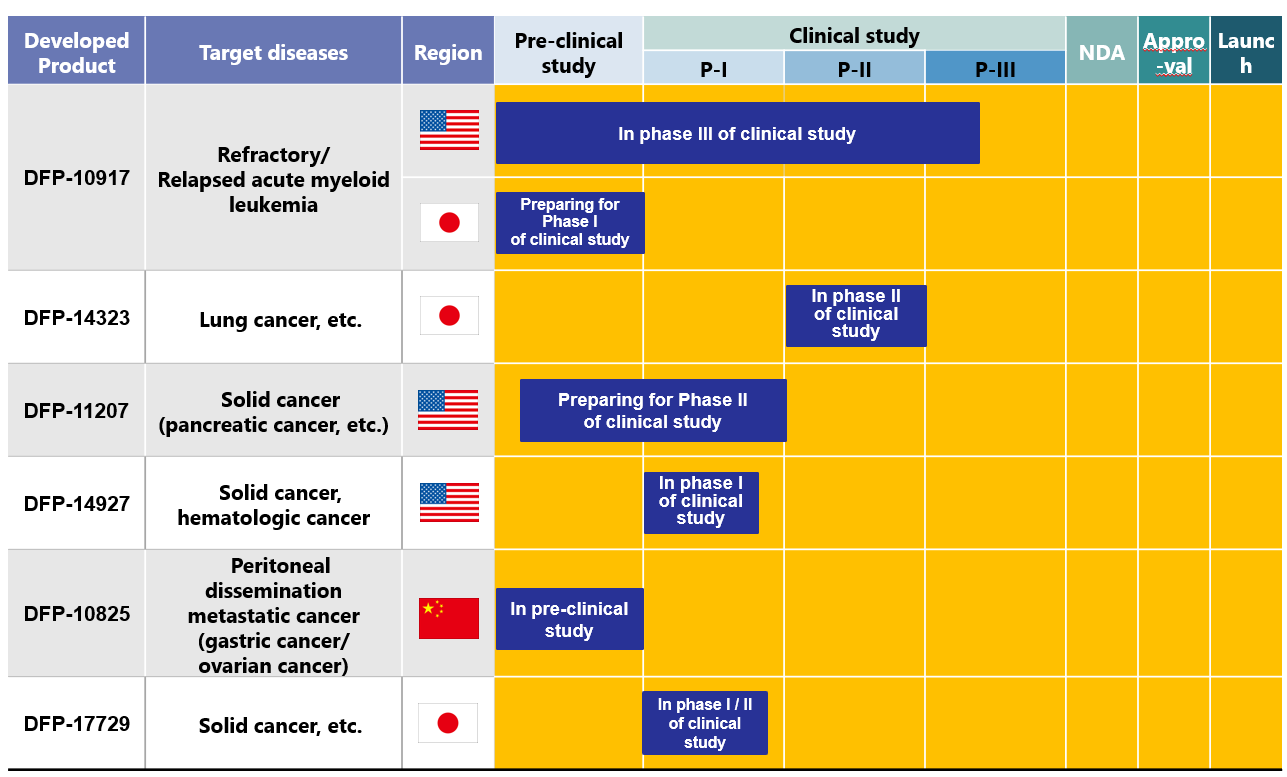
As of now, Delta-Fly Pharma has the following 6 drug pipelines in accordance with the above mentioned management policy.
The progress, current situation, and future plan for the development and commercialization of each pipeline are as shown below. Four candidate drugs are undergoing clinical studies. For the remaining two candidate drugs, clinical studies are being prepared.
(Taken from the reference material of the company)
1) 「DFP-10917」
Item | Outline |
Main target disease | Refractory and recurrent acute myeloid leukemia. The number of deaths associated with acute myeloid leukemia is 10 thousand in Japan, 30 thousand in the U.S., 30 thousand in Europe, and 20 thousand in China. 85% of the people who died from leukemia were 60 years old or older.
(Standard treatment methods have been established. About 70% of patients go into remission temporarily, as the cancer cells disappear from blood, but recurrence rate is high, and only 30% of patients can recover fully.) |
Characteristics of existing medicines, etc. | The existing medicine CNDAC is targeted at solid tumors. Dosage is high, and administration is conducted intravenously or orally in a short period of time. The efficacy against solid tumors is limited, and serious side effects were observed in some cases. |
Improved points and effects of modules | The dosage was reduced, and administration was conducted intravenously and continuously for a long period of time. As a result, there emerged different effects from those of conventionally used nucleic-acid derivatives (such as cytarabine and gemcitabine). It can be expected that the drug will be effective for the patients of refractory and recurrent acute myeloid leukemia, which cannot be treated with existing chemotherapy. The product excels in the balance between effectiveness and safety, and is optimal for the treatment of terminal hematologic cancer. |
Countries where patents were acquired (Nov. 2020) | Japan, the U.S., EU, China, Australia, South Korea, and Russia |
(State of development, and future commercialization)
In the clinical phase I/II tests carried out in the U.S., the drug was effective for 48% (14/29) of patients in the phase II, indicating high effectiveness. Taking this result, the company had a meeting with the U.S. Food and Drug Administration (FDA) after the clinical phase II test, and submitted a plan for the clinical phase III test. Consent was obtained by US FDA, however, as the treatment guideline of refractory and recurrent acute myeloid leukemia was changed. After the startup meeting with US FDA, the company re-submitted the revised protocol for the clinical Phase III study and the screening of research subjects started.
However, the effect of the spread of the novel coronavirus in the U.S. has not yet calmed down, and the patients’ enrollment has slowed down in such areas with large numbers of infected patients.
As a measure for promoting case registration, the company decided to increase the number of hospitals partaking in clinical trials, currently 25, and expand the target range of patients. Also, the current tests at the hospitals partaking in clinical studies will be continued, while consulting with the US FDA
Further, the drug substance and the final formulation for the new drug application of the DFP-10917 have been secured, and there are no changes in the policy of releasing the product in the U.S. market in fiscal 2022.
As for Japan, the licensee, Nippon Shinyaku Co., is preparing for the clinical phase I study. Also, the company received advice from Pharmaceuticals and Medical Devices Agency (PMDA).
Regarding the rights in territories outside Japan, negotiations for licensing contracts with pharmaceutical companies in Europe, the U.S., and China are in progress.
(Patent-related)
In May 2020, the company submitted a substance patent for the new derivative of Venetoclax, which is planned to be used with DFP-10917.
The new derivative of Venetoclax, for which the company submitted a patent, is a new substance acquired by forming a covalent bond between Venetoclax and a water-soluble polymer, and can selectively transport the active substance of Venetoclax to the targeted cancer cells; in experiments on animals transplanted subcutaneously human acute myeloid leukemia cells, it indicated similar results to the existing Venetoclax with less than a few tenths of the dosage while being safer.
2) 「DFP-14323」
Item | Outline |
Main target disease | Terminal stage lung cancer, etc. |
Characteristics of existing medicines, etc. | The existing medicine “Ubenimex” (UBX) is targeted at blood cancer. The dosage is high, and administration is conducted intravenously or orally with a single agent. It is indicated that the drug is for blood cancer only, but it showed a survival advantage against lung cancer. |
Improved points and effects of modules | For the purpose of enhancing the antitumor effect, the dosage was reduced, and the drug was used together with a molecular target drug. As a result, the efficacy against lung cancer was confirmed. The immune function in cancer patients is improved, and the effectiveness of existing drugs is enhanced. The drug is expected to treat terminal or elderly patients of solid tumors. |
Countries where patents were acquired (Nov. 2020) | Japan, the U.S., EU, Australia, Russia, Korea, Republic of China |
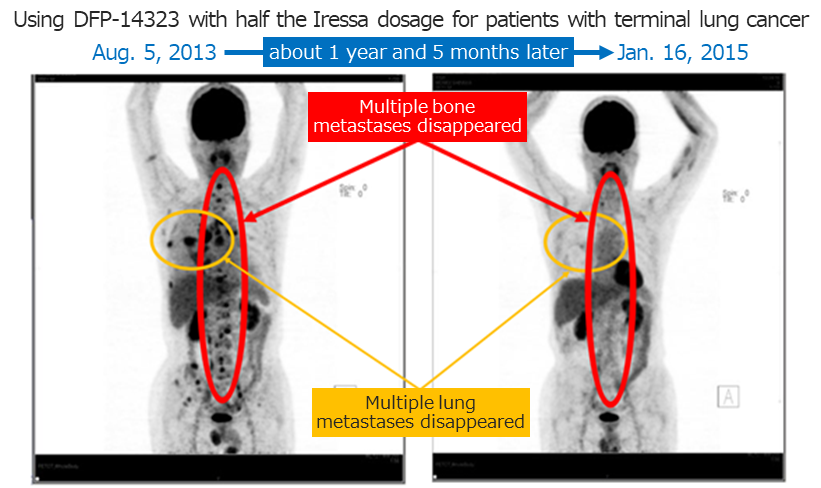
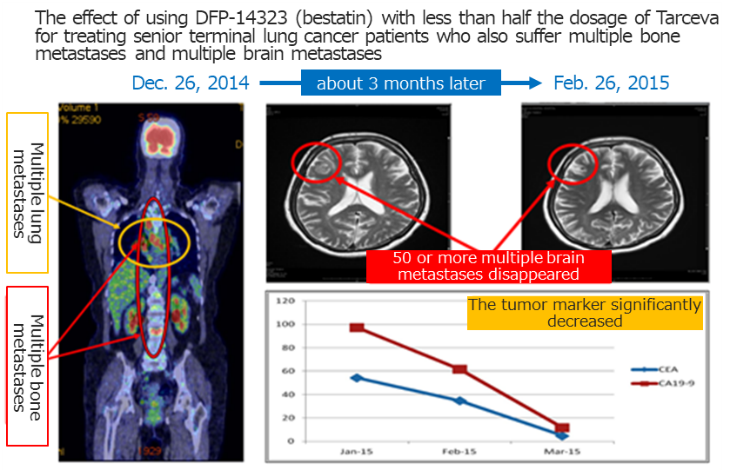
◎Confirming the clinical efficacy of DFP-14323
 |
 |
(Taken from the reference material of the company)
(State of development, and future commercialization)
As for the existing medicine Ubenimex, Nippon Kayaku Co., Ltd. obtained the approval for its efficacy and effect of “prolonging the survival period of adults suffering from acute non-lymphatic leukemia when combined with maintenance and intensive chemotherapeutic agents after remission” in Japan.
Delta-Fly started the clinical phase II study for the combined treatment of low-dose EGFR-TKI targeted at patients of EGFR gene mutation-positive non-small cell lung cancer as an additional indication in January 2018 in Japan and since the facilities that conduct the clinical studies have increased in Japan, the company proceeded with registering the new patients’ enrollment. In March 2020, the registration was complete for all patients’ enrollment.
DFP-14323 is the development code for obtaining approval as a Ubenimex’s new drug with expanded new indications.
Afterwards, during the effect measurement of the clinical phase II study based on the disease control rates (DCR) of all registered cases (including brain metastasis cases), a DCR of 100% was confirmed in June 2020, and efficacy was confirmed with the DCR of 100% and an overall response rate (ORR) of 65.4% or higher, in an effect measurement evaluation by independent medical doctors. The company thinks the product displayed excellent curative effects for brain metastases in patients of non-small cell lung cancer.
Further, based on the fact that it was discovered to be useful as a combination medicine for the treatment of patients of terminal non-small cell lung cancer with brain metastasis, the company made an international patent application to the member nations of the Patent Cooperation Treaty (PCT).
In addition, regarding the results of the clinical phase II test held in Japan, the company took part in the European Society for Medical Oncology (ESMO) Asia Congress 2020 held in November 2020, and got accepted for a poster presentation.
Based on the favorable results of the clinical phase II study held in Japan as well as its intellectual property foundation, the subjects for the clinical phase III comparative study for DFP-14323 are scheduled to be Non-small cell lung cancer patients with brain metastasis, and by including China, which is said to have the largest number of lung cancer patients in the world, the company is proceeding with preparations to obtain approval and release DFP-14323 as soon as possible.
If everything goes well, the company aims to obtain approval for additional indication in Japan, and release DFP-14323 by fiscal 2023.
The company signed a contract for an exclusive license right in Japan with Kyowa Chemical Industry Co., Ltd. (unlisted), but in November 2020, Kyowa Chemical Industry Co., Ltd. withdrew its plan for joint development and terminated the contract because of internal circumstances at Kyowa Chemical Industry.
The company will single-handedly continue pursuing the manufacture and sales approval of the generic drugs of Ubenimex from PMDA. Furthermore, the company will wait till around June 2021, for the evaluation of Progression Free Survival (PFS) and Overall Survival (OS), and apply to the PMDA for the manufacture and sales approval for the additional indication product for Ubenimex.
(Patent-related)
In May 2020, a patent was granted in Europe.
Currently, they have a pending patent application for DFP-14323 in the People's Republic of China and are following up on the evaluation process with the China National Intellectual Property Administration. When the patent is granted in the People's Republic of China, the company will have a foundation for expanding its business globally to major countries.
3) 「DFP-11207」
Item | Outline |
Main target disease | Solid tumors (such as pancreatic cancer) |
Characteristics of existing medicines, etc. | The existing medicine TS-1 has hematotoxicity, including the reduction of blood platelets, and it is difficult to continue treatment sufficiently. |
Improved points and effects of modules | DFP-11207 is a compound developed by combining three modularized active substances (modules I, II, and III) for sustained release, inhibition, and deactivation, in order to control the pharmacokinetics of 5-fluorouracil (5-FU), which has anticancer effects. It avoids hematotoxicity, including the decrease of blood platelets, which is caused by conventional 5-FU anticancer drugs, improves the balance between efficacy and safety, and enables long-time continuous treatment. This is a representative case of module drug development, in which the combination of compounds was improved. Optimal for preventing post-operation relapse or metastasis of micro cancer, and high life-prolongation effect can be expected. |
Countries where patents were acquired (Nov. 2020) | Japan, the U.S., EU, China, Australia, Korea, Russia, Republic of China, Hong Kong |
(State of development, and future commercialization)
In the U.S., the company proceeded with the clinical phase I study for solid tumors (digestive system cancer), and determined the recommended dose at the next test and confirmed that the decrease of blood platelets does not occur as a side effect, which has been caused by conventional 5-FU anticancer drugs.
Currently, the preparations are progressing as testing the effects of food has finished, and the company summarized the process, held a discussion with the clinical investigators, and formulated the plan for the clinical phase II study with the combined use of anticancer drugs.
The company announced the results of the clinical phase I study and the food effects’ study at the conferences of the Chinese Society of Clinical Oncology (CSCO) and Japan Society of Clinical Oncology (JSCO) in 2019.
Moreover, in May 2020, the result of the clinical Phase I study in the US was published in the American cancer treatment journal “Investigational New Drugs.” The drug’s safety was confirmed as it does not cause diarrhea or platelet toxicity, does not require a withdrawal period, and leukopenia is mild, and it was recognized as a drug that could have a life-extending effect.
The company is negotiating with Chinese pharmaceutical companies interested in these American clinical data to open up opportunities for joint development between the U.S. and China.
It aims to obtain approval and sell it in the U.S. or in China by fiscal 2024.
4) 「DFP-14927」
Item | Outline |
Main target disease | Pancreatic cancer, gastric cancer, and myelodysplastic syndromes |
Characteristics of existing medicines, etc. | The existing medicine DFP-10917 needs to be administered for 14 days in a row, by using a pouch for continuous intravenous injection, and it was necessary to improve its convenience. The target disease has been only blood cancer. |
Improved points and effects of modules | DFP-14927, a polyethylene glycol-conjugated candidate anticancer substance, is a polymeric delivery of DFP-10917. It selectively clusters around cancer tissue, and discharges DFP-10917 effectively inside cancer cells. The frequency of administration was reduced to once per week, and intravenous drip infusion was adopted. As a result, the medicine now can be used against solid tumors and myelodysplastic syndrome as well as blood cancer. Additionally, in animal models with pancreatic cancer, it was confirmed to be more effective and safer than gemcitabine, the standard chemotherapy for pancreatic cancer. |
Countries where patents were acquired (as of the end of Nov. 2020) | Japan, the U.S., China, Australia, Russia, Hong Kong |
◎Confirming DFP-14927 efficacy on animals
In animal models of pancreatic cancer, both the efficacy and safety of DFP-14927 were superior than gemcitabine, the standard chemotherapy for pancreatic cancer.
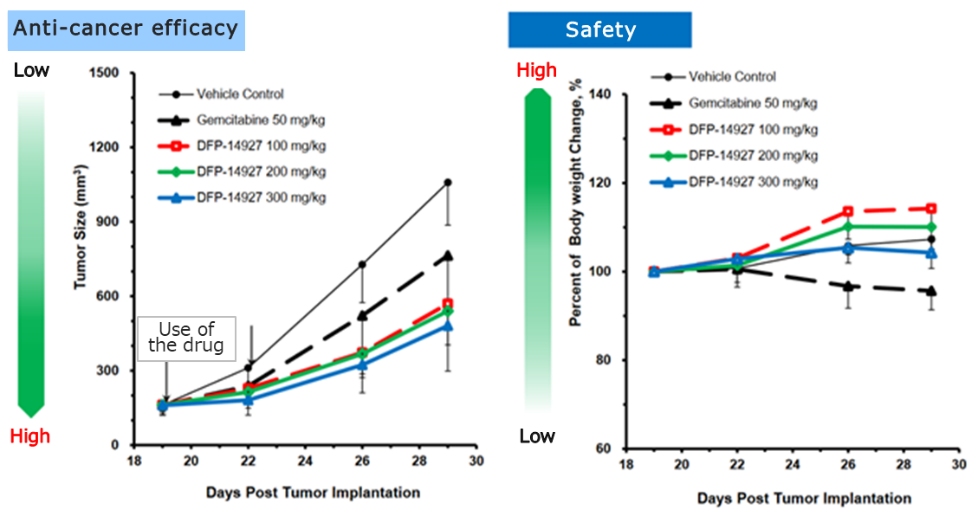
(Taken from the reference material of the company)
(State of development, and future commercialization)
The preclinical study has been completed in the U.S. The data of the preclinical study indicates that the level of the medicine in blood is stable for a long period of time when it is administered once a week, and that there is the antitumor effect against solid tumors.
In March 2018, the company concluded a contract for collaborative development with Sanyo Chemical Industries, Ltd. and prepared for the application for the start of the clinical phase I study, and on January 18, 2019, the U.S. FDA completed the examination of the safety of Investigational New Drug (IND), and approved the clinical phase I study in the U.S. And the company started clinical phase I study aimed at patients with digestive system cancer including pancreatic cancer and gastric cancer.
Due to the effect of the spread of the novel coronavirus, the case registration has slowed down in areas with large numbers of infected patients, but once the safety around the present dosage is confirmed, the company plans to select the optimal cancer, add multiple major cancer centers in the U.S., and shift to expanded tests corresponding to the clinical phase II study. Further, the company plans to discuss the possibility for the clinical phase I/II studies for Myelodysplastic Syndrome (MDS) for hematologic cancer.
They aim to obtain approval and start sales in the U.S. by FY 2025.
Regarding distribution rights in territories outside Japan, negotiations for licensing contracts with pharmaceutical companies in Europe, the U.S., and China are in progress.
5) 「DFP-10825」
Item | Outline |
Main target disease | Gastric cancer, ovarian cancer, and peritoneal metastasis from pancreatic cancer |
Characteristics of existing medicines, etc. | Although the basic drug siRNA has a definite inhibitory effect as its basic effect, its clinical effect in systemic administration has been poor. |
Improved points and effects of modules | Nucleic acid drugs using RNA interference are expected to be the next cancer treatment drugs next to molecular-targeted cancer drugs and cancer immunotherapeutic drugs. The nucleic acid drug DFP-10825 is designed to be effective by intraperitoneal rather than systemic administration, as it specifically inhibits the factors that significantly affect cancer growth by RNA interference. In patients with ovarian cancer or stomach cancer, fluid retention such as pleural fluid and ascites (peritoneal metastasis) is observed at the terminal stage, but ascites is controlled by injecting the drug directly into the abdominal cavity to exert an effect. It is expected to relieve the pain and lead to the patients’ prolonging life. |
Countries where patents were acquired (Nov. 2020) | Japan, the U.S., EU, China, Korea, Russia, Republic of China, Hong Kong |
(State of development, and future commercialization)
The company has already completed efficacy and pharmacokinetics studies against peritoneal metastasis that causes ascites associated with ovarian, stomach or pancreatic cancer. Preliminary investigations based on the current Good Manufacturing Practice (cGMP) standards have also been completed for the manufacture of the clinical study drugs, such as drug substances, DDS and preparations. From now on, after adding preclinical studies according to the Good Laboratory Practice (GLP) standards for conducting non-clinical studies concerning safety of drugs using a part of the funds obtained from the stock listing, the company is planning to apply for IND to the US FDA and will begin the clinical phase I study for peritoneal metastasis of ovarian, stomach or pancreatic cancer patients in the U.S. The company has already received each country’s patent certifications.
While making preparations for the drug substance and formulation for clinical studies, preclinical studies using animals are being conducted, and the company aims to start the clinical studies in the U.S. or Japan by fiscal 2020.
6) 「DFP-17729」
Item | Outline |
Main target disease | Terminal stage pancreatic cancer, malignant gastric lymphoma, gastric cancer, and lung cancer. |
Characteristics of existing medicines, etc. | Urinary alkalinizing agents, which are existing drugs, are targeted for hyperuricemia and others, but it has been confirmed that they provide a life-prolonging effect in pancreatic cancer and have an antitumor effect on each cancer tumor. |
Improved points and effects of modules | Normal cells are more alkaline outside the cells than inside the cells, but cancer cells are more acidic outside the cells. This is because the growth of cancer cells promotes glycolysis, producing lactic acid and hydrogen ions, and they are actively released into the extracellular space. DFP-17729 suppresses the growth of cancer by alkalizing the outside of cancer cells. In other words, it cleans the area surrounding the cancer, and calms the cancer down. It has been confirmed in animal experiments that the combined use of an anticancer drug and an immune checkpoint inhibitor enhances the effect as compared with the monotherapy with an immune checkpoint inhibitor. |
Countries where patents were acquired (as of the end of Nov. 2020) | Japan, Korea |
◎Confirming the clinical efficacy of DFP-17729
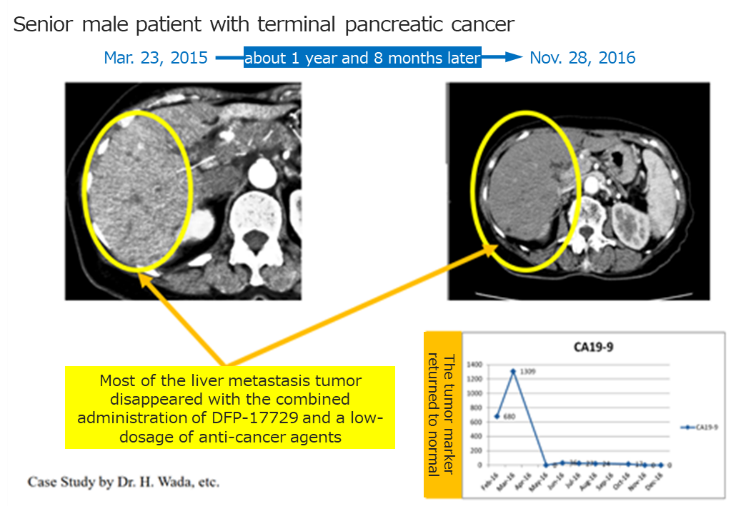 |
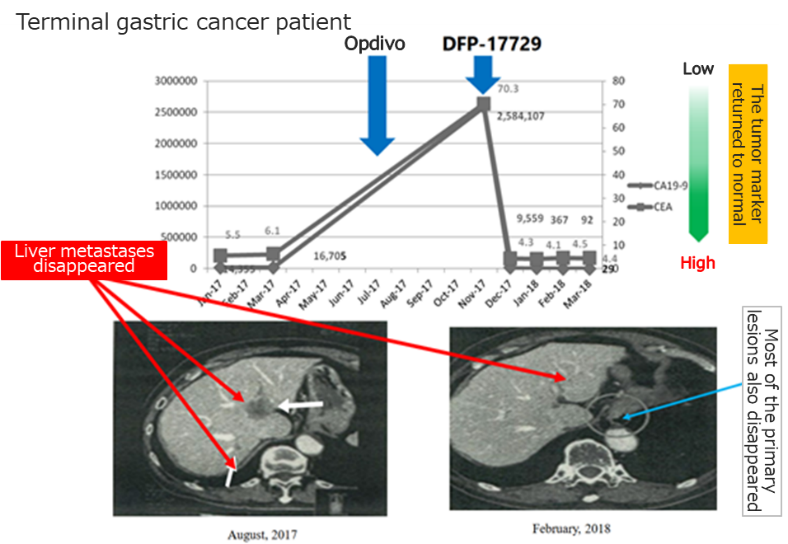 |
(Taken from the reference material of the company)
(State of development, and future commercialization)
The company is preparing for the additional indication of urine alkalizing agents, which are approved and sold as pharmaceutical products, as anti-cancer drugs in Japan.
Because urine alkalizing agents are already being used in clinical practices for the efficacy and effect of “acidosis improvement” to treat “hyperuricemia” and “tumor lysis syndrome,” so the preclinical study is not necessary.
The company aims to expand the range of anti-tumor effects of existing drugs through the combined use of anti-cancer agents and an immune checkpoint inhibitor and provide new cancer treatments. It also aims to start the clinical studies in Japan by FY 2020.
In March 2020, the company signed a licensing contract with Nippon Chemiphar Co., Ltd., by which it agreed to give Nippon Chemiphar Co., Ltd., the exclusive marketing right and the exclusive manufacturing right of DFP-17729 in Japan.
Delta-Fly Pharma will perform clinical studies of the combined use with existing anti-cancer agents for pancreatic cancer patients, while Nippon Chemiphar Co., Ltd. will be responsible for manufacturing and selling DFP-17729 in Japan after the PMDA approval.
In May 2020, the company submitted a paper about DFP-17729 and it’s accepted by the journal of American Association for Cancer Research “Molecular Cancer Therapeutics.”
Generally, the 5-year survival rate of pancreatic cancer patients is less than 10%, which is severely low. However, this research indicates that it does not only increase the efficacy of existing pancreatic cancer treatments, but also increase the effectiveness of an immune checkpoint inhibitor (anti-PD-1 antibody). Moreover, DFP-17729 does not show any of the side effects of the existing anti-cancer drugs and it was confirmed that it does not produce extra toxicity from combining it with existing anti-cancer drugs.
After obtaining these results, the company submitted a clinical trial plan to the PMDA in July 2020 with the aim of executing the clinical phase I/II studies in multiple medical institutions in Japan, targeting patients with terminal pancreatic cancer, and received permission for the execution of the studies after completion of an examination by PMDA.
Taking the condition of the terminal pancreatic cancer patients into account, the clinical study will be used to investigate and confirm the safety/effectiveness of the clinical phase I/II studies before transitioning into the clinical phase III study. The clinical phase I study will confirm the safety when using the existing drugs and DFP-17729 at the same time, and the clinical phase II study will be a comparative test to confirm whether DFP-17729 excels compared to existing drugs.
In accordance with the agreement with PMDA, the company plans to execute the tests in six institutions including university hospitals and major cancer centers in the Kanto region, and since the in-hospital examinations by each Institutional Review Board (IRB) were completed in the three major hospitals in the Kanto region responsible for the clinical phase I study, the company formed a clinical study execution contract with each hospital. The patients’ enrollment for the clinical phase I study began on November 8, 2020.
Further, the company plans to enter the clinical phase II study in the six major hospitals in the Kanto region after the confirmation of its safety by the clinical phase I study for terminal pancreatic cancer patients. Presently, the clinical phase I/II studies have not been affected by the spread of the novel coronavirus. After proceeding with the clinical study in Japan in collaboration with the company’s partner, Nippon Chemiphar Co., Ltd., the company plans to expand into Europe, the U.S., and various Asian countries in the future, based on the clinical study data obtained in Japan.
1-5 Four characteristics as a bio-venture
The company as a bio-venture has the following four main characteristics.
1) Module drug development
As described above, the company is patenting existing drugs, etc. by re-inventing them with ingenuity based on “modules” (components) and creating new drugs with improved balance between clinical efficacy and safety.
2) Specialized in the development of anti-cancer drugs
By working specifically on “anti-cancer drugs,” which still have limited effectiveness and cause various side effects, the company is accelerating the development of new drugs through module drug development and contributing to the improvement of the social life of cancer patients.
3) Development by experienced members
The development members consisting of people who have been engaged in research and development of anti-cancer drugs for many years at pharmaceutical companies and clinicians who are familiar with cancer patients advance the development of drugs with certainty and meet unmet medical needs. This sharply differentiates the company from others, giving competitive advantage.
4) Effective utilization of external resources
The company operates efficiently by focusing on management and operation of research and development without having factories or research institutes and proactively cooperating with external contractors and other organizations for outsourcing tasks.
2. Earnings Trends
2-1 The second quarter of Fiscal Year ending March 2021 Earnings Results
1) Earnings trends
| 2Q of FY 3/20 | 2Q of FY 3/21 | YoY |
Operating Revenue | - | 100 | +100 |
Operating Cost | 725 | 563 | -162 |
R&D Expense | 598 | 422 | -175 |
Other SG&A expenses | 127 | 140 | +13 |
Operating Income | -725 | -463 | +262 |
Ordinary Income | -729 | -463 | +266 |
Quarterly Income | -731 | -464 | +266 |
Unit: Million yen
(Operating Revenue)
The company recorded milestone revenue related to the licensing contract with Nippon Chemiphar Co., Ltd., during the second quarter of the term (July to September).
(Operating Cost)
The number of Medical institutions undertaking the clinical studies for pipelines in development, and the number of patients’ enrollment were increased, and progress was made in the preparation for the new clinical studies, but the R&D expense decreased by 175-million-yen, year on year.
(Operating income)
Operating loss shrank 262-million-yen year on year to 463 million yen.
2) Financial Conditions and Cash Flows
◎Main BS
| End of Mar. 2020 | End of Sep. 2020 |
| End of Mar. 2020 | End of Sep. 2020 |
Current Assets | 2,115 | 1,610 | Total Liabilities | 105 | 66 |
Cash | 1,943 | 1,582 | Total Net Assets | 2,056 | 1,591 |
Noncurrent Assets | 46 | 47 | Retained Earnings | -3,622 | -4,087 |
Property, Plant and Equipment | 43 | 42 | Total Liabilities, Net Assets | 2,162 | 1,658 |
Total Assets | 2,162 | 1,658 | Balance of Short and Long-Term Debts | 5 | 2 |
Unit: Million yen
Equity ratio was 96.0%, 0.9 points up year-on-year.
◎Cash Flows
| 2Q of FY 3/20 | 2Q of FY 3/21 | Increase/Decrease |
Operating cash flow | -735 | -356 | +379 |
Investing cash flow | -13 | -0 | +12 |
Free cash flow | -748 | -356 | +391 |
Financing cash flow | 103 | -3 | -106 |
Cash and Equivalents at the end of term | 2,862 | 1,582 | -1,280 |
Unit: Million yen
The cash position declined.
2-2 Fiscal Year ending March 2021 Earnings Forecasts
| FY 3/20 | FY 3/21 (Estimate) | YoY |
Operating Revenue | 100 | 300 | +200 |
SG&A | 1,645 | 1,150 | -495 |
R&D Expenses | 1,397 | 880 | -517 |
Other SG&A Expenses | 248 | 270 | +22 |
Operating income | -1,545 | -850 | +695 |
Ordinary income | -1,552 | -850 | +702 |
Net loss | -1,555 | -850 | +705 |
Unit: Million yen
There is no change in the full-year forecast. Restrictions on quarantine due to the spread of the novel coronavirus also affected visits to medical institutions for participants of the clinical studies, and major Japanese pharmaceutical companies temporarily suspended the starting-up of new clinical trials and patient enrollment in ongoing clinical trials, resulting in a 57% drop in new patients’ enrollment in the first half of April compared to March, the company steadily advanced its clinical development despite the impact.
(Operating Revenue)
The company expects to make 300 million yen of milestone compensation for licensing contracts.
As for business revenue, considering the uncertainties of the progress of clinical tests and license negotiations, they thought that posting milestone compensation and lump-sum contract payment to be expected would not be appropriate at this stage. They plan to clarify the outlook when revenue is secured.
(Operating Cost)
As the company had ordered to manufacture active pharmaceutical ingredients and preparations to a CMO in the previous term earlier than planned, it expects R&D expenses to decrease.
3. Growth Strategy
The company will firmly continue the development of the 4 products undergoing the clinical studies and the 2 products undergoing preparation for the clinical studies, and from FY 2022, it aims to steadily release them to the market. Further, the company plans to expand profitability and focus on securing alliance partners in Japan, China, Europe, and the U.S.
Out of the six products presently in development, the product with the largest potential market scale is DFP-11207 with 100 billion yen. According to company estimates, the first product scheduled to be released in the market, DFP-10917, also has a market scale of 70 billion yen outside Japan, and 10 billion yen in Japan, a total of 80 billion yen.
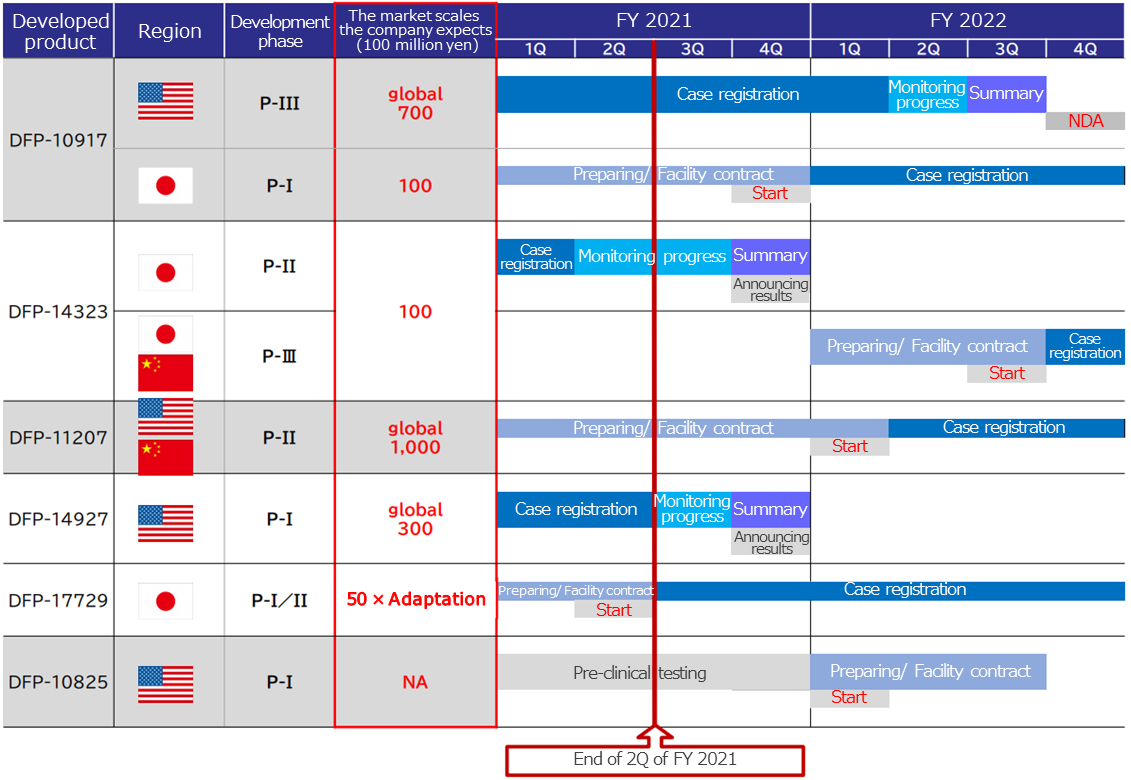
(Taken from the reference material of the company)
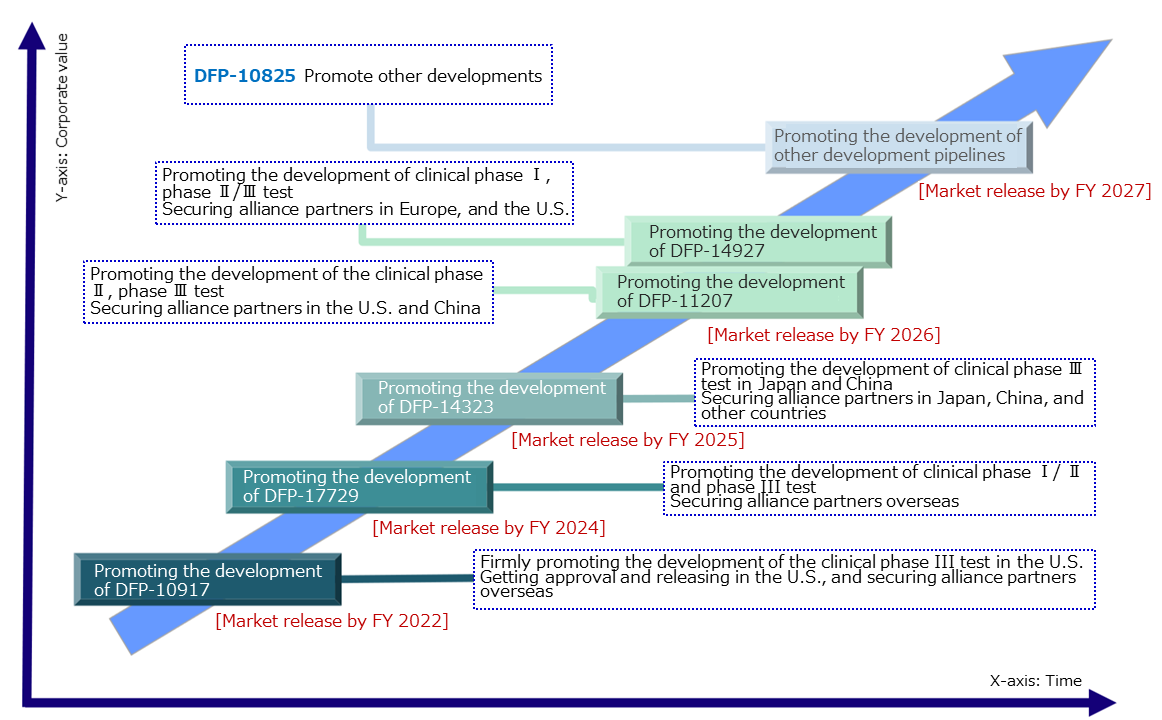
(Taken from the reference material of the company)
4. Conclusions
The spread of the novel coronavirus seemed to have slowed down the registration of cases for the clinical studies of DFP-10917 and DFP-14927 in the U.S., but there seems to be no major impact as of now. On the other hand, while the market scale is medium-sized, steady progress is being made in development and commercialization of DFP-14323, which is to be the third product to enter the Chinese market by fiscal 2025. DFP-14323 entered the preparation stage for its clinical phase III study. Progress is also being made in negotiations with their partner, and we have high expectations regarding the release of DFP-14323.
<Reference: Regarding Corporate Governance>
◎Organization type, and the composition of directors and auditors
Organization type | Company with audit and supervisory board |
Directors | 8 directors, including 4 outside ones |
Auditors | 3 auditors, including 2 outside ones |
◎Corporate Governance Report
The latest update: July 1, 2020.
<Basic policy>
Our company thinks that our mission is to operate our business while putting importance on the benefits of all stakeholders, including shareholders, clients, business partners, employees, and local communities, under the mission of “To provide treatment methods recommendable for cancer patients and their families with peace of mind through module drug development.” To accomplish this, it is indispensable to develop our business stably and perpetually. Our basic policy for corporate governance is to improve systems for securing the soundness, transparency, and efficiency of business administration, which will become the base for the above-mentioned development.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code>
It is written that “We follow all of the basic principles.”
This report is intended solely for information purposes and is not intended as a solicitation for investment. The information and opinions contained within this report are made by our company based on data made publicly available, and the information within this report comes from sources that we judge to be reliable. However, we cannot wholly guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration. Copyright(C) 2020 Investment Bridge Co., Ltd. All Rights Reserved. |


