Bridge Report:(4598)Delta-Fly Pharma Fiscal Year ended March 2021
 President Kiyoshi Eshima | Delta-Fly Pharma, Inc.(4598) |
 |
Corporate Information
Exchange | TSE Mothers |
Industry | Pharmaceutical products (manufacturing industry) |
President | Kiyoshi Eshima |
Address | 37-5 Nishikino, Miyajima, Kawauchi-cho, Tokushima-shi, Tokushima |
Year-end | End of March |
URL |
Stock Information
Share Price | Shares Outstanding | Total Market Cap | ROE(Actual) | Trading Unit | |
¥1,521 | 5,314,600 shares | ¥8,083million | -41.7% | 100 shares | |
DPS(Estimate) | Dividend Yield (Estimate) | EPS(Estimate) | PER(Estimate) | BPS(Actual) | PBR(Actual) |
¥0.00 | - | ¥-239.87 | - | ¥390.87 | 3.9 times |
*Share price is as of closing on June 4. Each figure was taken from the financial results for the fiscal year ended March 2021.
Earnings Trends
Fiscal Year | Net Sales | Operating Income | Ordinary Income | Net Income | EPS | DPS |
Mar. 2018 (Actual) | 150 | -243 | -244 | -246 | -71.20 | 0.00 |
Mar. 2019 (Actual) | - | -592 | -671 | -673 | -170.16 | 0.00 |
Mar. 2020 (Actual) | 100 | -1,545 | -1,552 | -1,555 | -348.32 | 0.00 |
Mar. 2021 (Actual) | 300 | -852 | -859 | -862 | -187.34 | 0.00 |
Mar. 2022 (Estimate) | 100 | -1,300 | -1,300 | -1,300 | -239.87 | 0.00 |
*Unit: million-yen, yen
*The estimated values were provided by the company. 500-for-1 share split was conducted on Jun. 25, 2018. EPS is adjusted retroactively.
This report introduces earnings trends, progress of the development etc. of Delta-Fly Pharma, Inc.
Table of Contents
Key Points
1. Company Overview
2. Earnings Trends
3. Growth Strategy
4. Conclusions
<Reference: Regarding Corporate Governance>
Key Points
- The company develops anticancer drugs under the original concept of “module drug development,” which means that they develop new anticancer drugs that have an improved balance between clinical efficacy and safety with fewer side effects, by using the existing active substances with anticancer property as “modules (components)” and designing dosage and administration, combination methods, etc. with ingenuity.
- “Module drug development” has benefits for patients, including the improvement of treatment effects, the reduction of side effects and costs, and also benefits for development, including the high exclusiveness in patenting, the swiftness of development, and low development risk. The company currently has 6 drug pipelines, and 4 candidate drugs are under clinical studies, and for the other 2 candidate drugs are being prepared for the clinical studies.
- In addition to module drug development, the company is characterized by the specialization in development of anticancer drugs, the development by experienced members, and efficient business operation utilizing external resources.
- Operating revenue in the term ended March 2021 was 300 million yen, up 200 million yen year on year, which was from milestone income received through the licensing agreements with Nippon Chemiphar Co., Ltd. and Nippon Shinyaku Co., Ltd. By increasing the number of medical institutions for the clinical study, the patients’ enrollment was accelerated. In the meantime, active pharmaceutical ingredients were manufactured as the preparation of investigational products for next studies. Operating loss decreased 693 million yen year on year to 852 million yen.
- Operating revenue for the term ending March 2022 as milestone compensation for licensing agreements is estimated at 100 million yen, down 200 million yen year on year. In addition to revenue from milestone compensation for DFP-10917, the company is expected to earn revenue, such as upfront payment through alliances with new partners, as the clinical studies of several candidate compounds for anticancer drugs, including DFP-10917 which is undergoing the clinical phase III study in the U.S. and DFP-14323 which is going through the clinical phase II study in Japan. The company plans to announce its future outlook in a timely manner when revenue is finalized. Operating cost is projected to grow 250 million yen year on year to 1.4 billion yen. R&D expense is expected to rise 223 million yen year on year so that the company can make steady progress with each of the pipelines under development. Operating loss will stand at 1.3 billion yen, up 447 million yen year on year.
- DFP-10917, which well be expected to launch as the first commercial product, has been given an international nonproprietary name of Radgocitabine. Although the company estimates the maximum sales volume of DFP-10917, Radgocitabine, to be 80 billion yen (70 billion yen from the global market and 10 billion yen from the Japanese market), the market size of acute myeloid leukemia (AML) will be larger than expected. We would like to keep an eye on information released by the company regarding the progress with the clinical phase III study, which is being carried out this fiscal year, before the company submits an application and launches Radgocitabine in the U.S. in the term ending March 2023 as planned.
1. Company Overview
Delta-Fly Pharma upholds the corporate ethos: “To provide treatment methods recommendable for cancer patients and their families with peace of mind, by diagnosing all states of cancer patients rather than focusing on only cancer,” and develops anticancer drugs under the original concept of “module drug development,” which means that they develop new anticancer drugs that have an improved balance between clinical efficacy and safety with fewer side effects, by using the existing active substances with anticancer property as “modules (components)” and designing dosage and administration, combination methods, etc. with ingenuity.
1-1 Corporate history
The President Eshima, who was born in Tokushima Prefecture, graduated from Nagoya Institute of Technology, completed the master’s course of Tokyo Institute of Technology, and joined the Otsuka Group, a pharmaceutical company in Tokushima Prefecture, which is his hometown. Then, he was assigned to TAIHO Pharmaceutical Co., Ltd., which is a business company of the Otsuka Group.
Immediately after joining the company, he was dispatched to Faculty of Science and Engineering, Waseda University, and engaged in the development of pharmaceuticals, especially new medicines composed of functional polymers, as a researcher for about 12 years. When he was in the section that seeks seeds of pharmaceutical products in TAIHO Pharmaceutical, he saw how the business administration of U.S. bio ventures was carried out. That stirred his willingness to become independent, manage a pharmaceutical company by himself, and create medicines with a new approach, rather than engaging in development in the R&D section of a leading pharmaceutical company. He also aimed to develop a business while not only creating medicines, but also considering what he can do for patients in front of him. In 2010, when he was 61 years old, he resigned from TAIHO Pharmaceutical, and established Delta-Fly Pharma. The company is committed to the development of anticancer drugs with fewer side effects and friendly to patients through module drug development. As of September 2020, the company has 6 drug pipelines.
It was listed in Mothers of Tokyo Stock Exchange in October 2018.
1-2 Corporate ethos and management philosophy
The corporate name “Delta-Fly” is derived from a “dragonfly.” Since dragonflies only go forward, and do not go backward, they represent the unflagging spirit, and they are also called “winning insects.” Namely, the corporate name implies the firm resolve to develop pharmaceutical products.
Corporate ethos | To provide treatment methods recommendable for cancer patients and their families with peace of mind, by diagnosing all states of cancer patients rather than focusing on only cancer, |
As mentioned later, the company considers that its social mission is not to develop anticancer drugs only for eradicating “cancer,” but to provide anticancer treatment with reasonable price while curbing side effects, which are serious issues with anticancer drugs, so that patients and their family members can use it without worry.
1-3 Environment surrounding the company
According to “the demographic statistics in Japan in 2018” published by the Ministry of Health, Labor and Welfare, the mortality rate (number of deaths per 100,000 people) of malignant neoplasm (cancer) was the highest: 298.3 in 2016. It has been the highest for over 30 years since it replaced cerebrovascular disease, whose mortality rate was 134.3 while that of malignant neoplasm was 142.0, in 1981. It is increasing year by year.
It is said that the incidence of cancer is growing due to the aging of the population, the change in lifestyles, including dietary habits, etc.
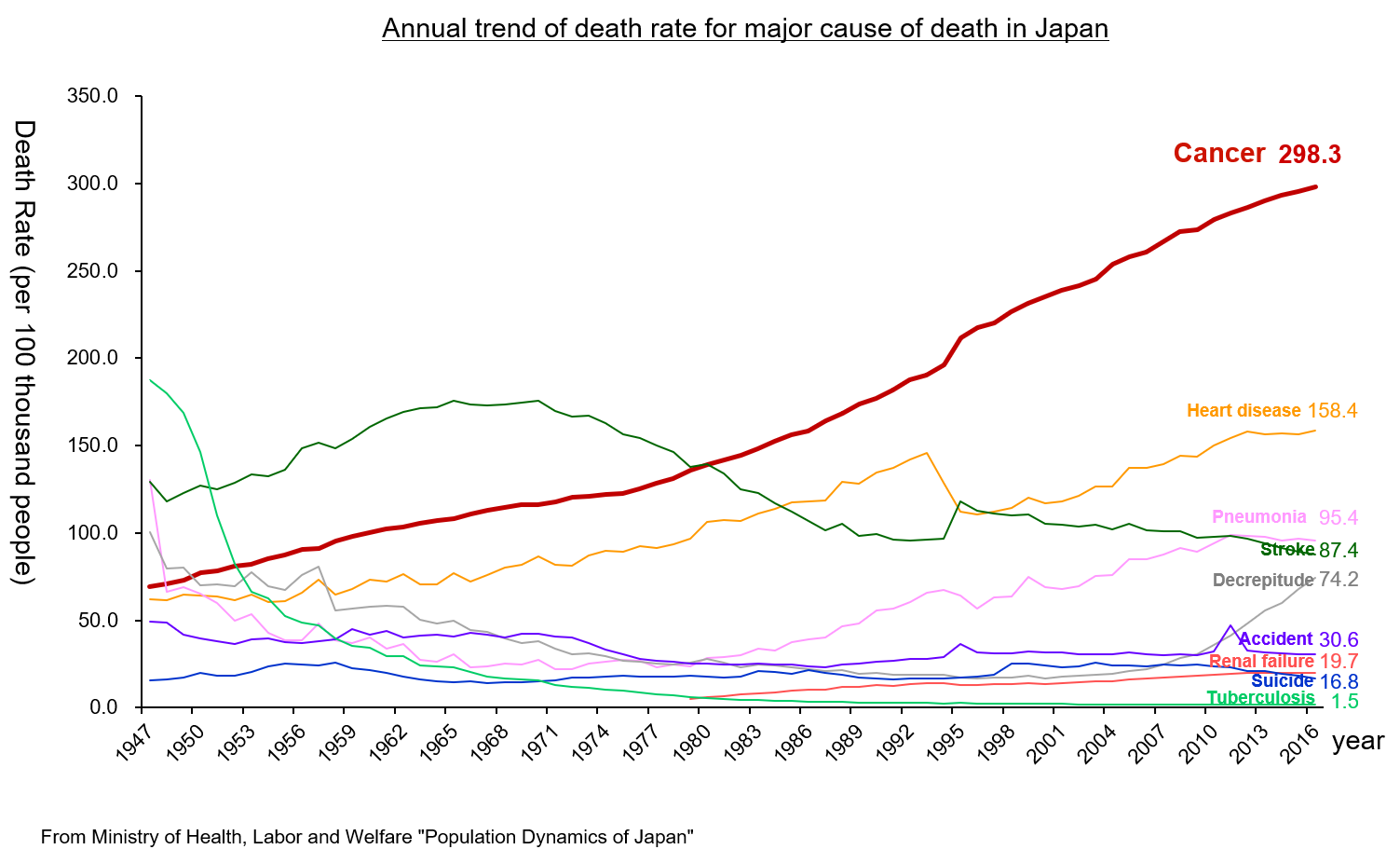
(Taken from the reference material of the company)
(Taken from the reference material of the company)
In these circumstances, various anticancer drugs are used, and new medicines are being developed. But, as publicly known, the side effects of anticancer treatment are significant, so there are considerable needs for the reduction of side effects from the viewpoint of improving the quality of life (QOL) of patients.
(Mechanism of side effects)
Since cancer cells rapidly divide and proliferate, anticancer drugs are designed to kill rapidly growing cancer cells. However, anticancer drugs affect not only cancer cells, but also the normal cells that rapidly divide, such as blood cells produced in bone marrow, the cells of digestive organs, the cells of genitals, and hair root cells, causing side effects, such as nausea, vomiting, hair loss, and fatigue.
1-4 Business contents
1-4-1 Delta-Fly Pharma’s method for creating medicines: Module drug development
What distinguishes the company most among a lot of bio ventures is its concept for developing medicines: “module drug development.”
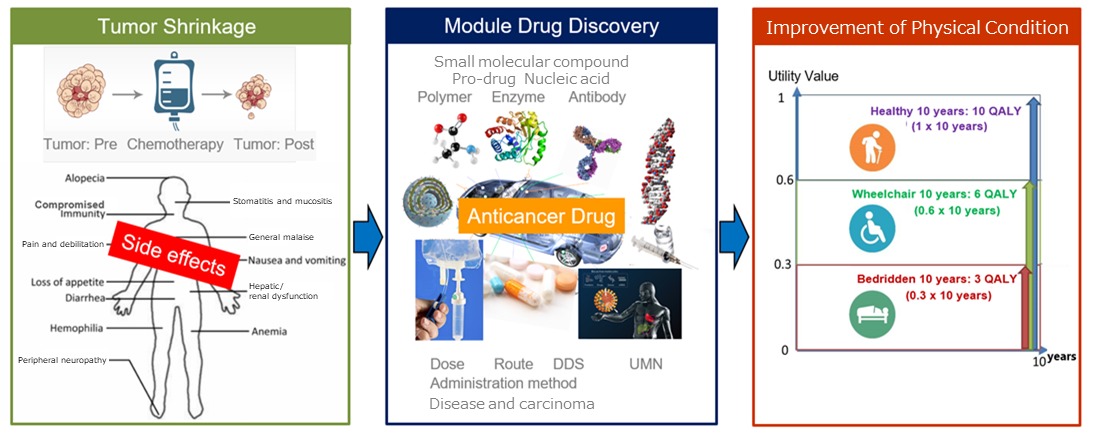
(Taken from the reference material of the company)
“Module drug development” means the development of new anticancer drugs that have an improved balance between clinical efficacy and safety by using the existing active substances with anticancer property as “modules (components)” and designing dosage and administration, combination methods, etc. with ingenuity.
Through “module drug development,” the company focuses on not only “cancer circumstances” but also the whole conditions of “cancer patients,” improves the anticancer drugs with fewer side effects and which have various side effects in a multifaceted manner, and produces medicines whose side effects are so fewer that you can recommend them to cancer patients and their families.
(Advantage of module drug development)
Merits for patients | ・Since medicines are created based on data on patients, treatment effects are expected to improve.・Since medicines are created based on data on patients, conventional side effects are expected to reduce. ・The number of fundamental and clinical tests is small and their periods are short; accordingly, their costs are not considerable. |
Merits for development | ・Since medicines can be patented due to novelty and inventive steps, they will have high exclusivity.・Since medicines are developed based on data on patients, development speed is high. ・Since medicines are developed based on data on patients, development risk is low. |
In general creation of anticancer drugs, chemicals that act on the cancer-specific parts are extracted at the stage of fundamental search and research, and possible chemicals become candidates for anticancer drugs. However, it is necessary to check their functions at the clinical stage and demonstrate efficacy and safety through clinical tests. Accordingly, the R&D period from the basic stage is long.
In contrast, “module drug development” does not require fundamental search or research so much, because the active substances of already used anticancer drugs are combined, and it is possible to predict efficacy and safety at the clinical stage. Accordingly, it is possible to start clinical tests in one to two years after the start of medicine development. Like this, compared with general development of anticancer drugs, the R&D is more efficient, the development period is shorter, and the risk of development, including the failure in clinical tests, is lower.
In addition, when focusing on the issues with the treatment of cancer patients, the combination of off-patent pharmaceutical products by utilizing the knowledge and know-how of anticancer drugs enables them to be patented as new anticancer drugs.
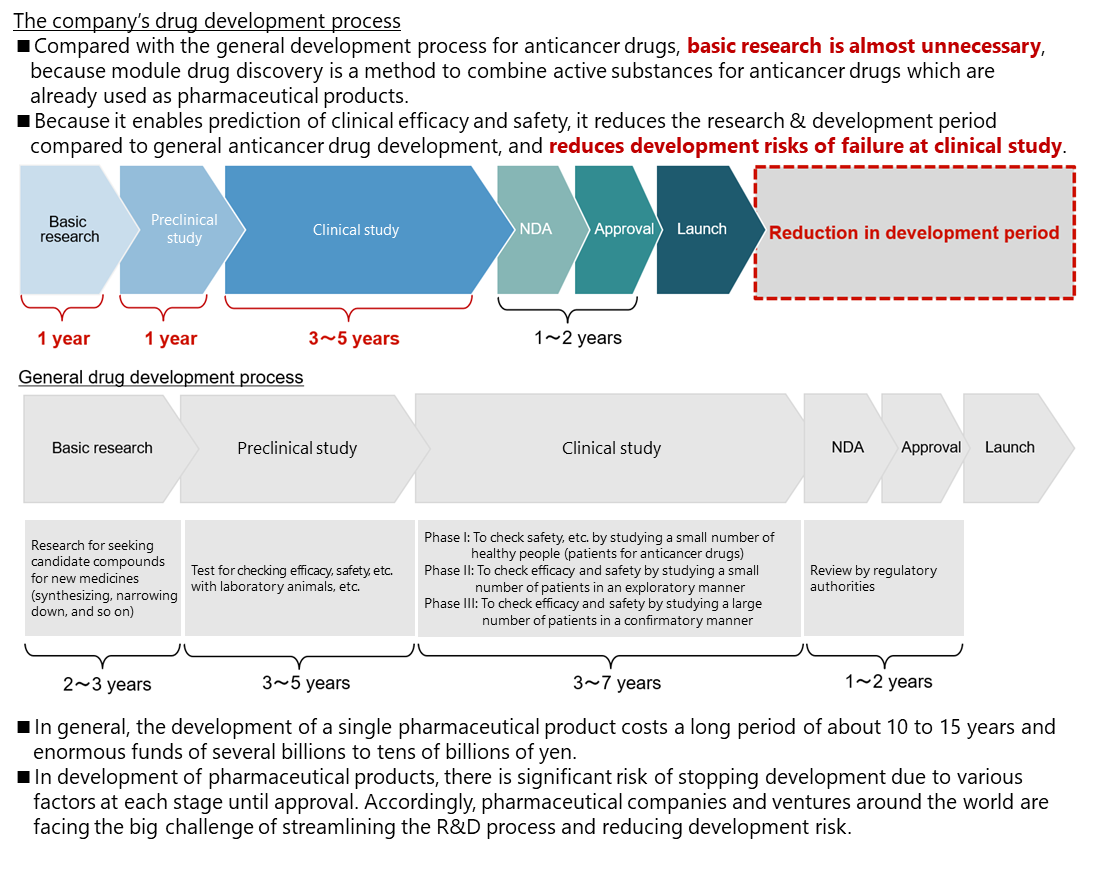
(Taken from the reference material of the company)
Nowadays, an increasing number of pharmaceutical companies engage in drug-repositioning activities to discover new effects of generic and existing medicines, for the purpose of reducing the cost for new drug development.
These are the same as “module drug development” in that existing medicines are used. It is difficult to patent these drugs based on generic medicines and drug repositioning because of the lack of novelty and inventiveness. On the other hand, “module drug development” will make all developed drugs patented. This is a defining difference.
As long as they try to solve the problems with anticancer drugs, they can create totally new anticancer drugs. Therefore, the company is certain that “module drug development” will bring significant innovation to methods for creating medicines.
1-4-2 Business and revenue models
(Business model: to develop an efficient R&D system)
Before a new pharmaceutical product is released, it is common that “fundamental research” is first conducted, “preclinical tests (tests for checking the pharmacological actions, in-vivo kinetics, harmful effects, etc. by using animals)” and “clinical tests (scientific tests for studying the effects of pharmaceutical products, treatment technologies, etc. on human bodies)” are carried out, applications are submitted to authorities to obtain approvals, products are manufactured, and then surveys are conducted after manufacturing, marketing, and sale.
In these processes, Delta-Fly Pharma concentrates on the management of R&D, while outsourcing meticulous tasks to excellent external R&D companies and manufacturers inside and outside Japan. The company has actualized an efficient R&D system in cooperation with external cooperative institutions according to development phases. It also engages in the R&D for new anticancer drugs by using a drug delivery system in collaboration with Sanyo Chemical Industries, Ltd. (1st section of TSE; 4471).
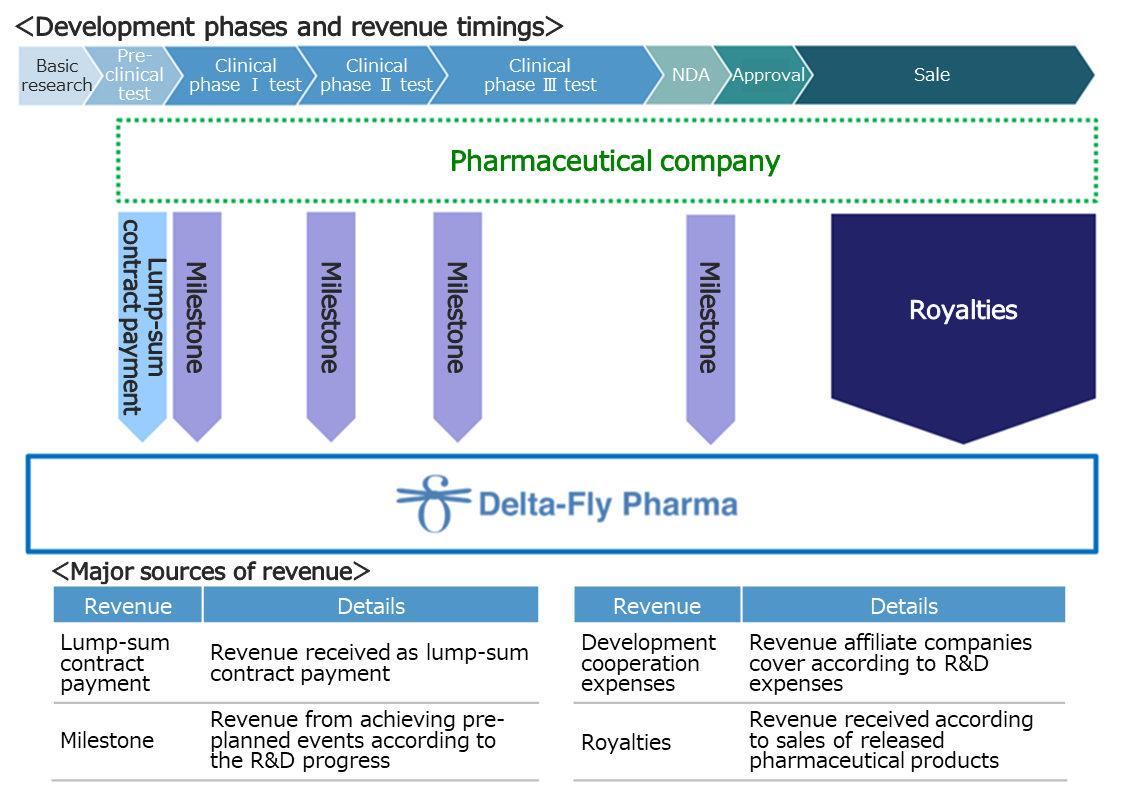
(Revenue model)
At the R&D stage, the main revenue sources are “lump-sum contract payment” for contracts with affiliated pharmaceutical companies, “milestone,” and “cooperation funds for development.” If collaborative products are released, the company will receive royalties according to sales.
Currently, Delta-Fly Pharma collaborates with the following two pharmaceutical companies.
Nippon Shinyaku Co., Ltd. (1st section of TSE, 4516) | Signed a contract for an exclusive license right for DFP-10917 in Japan. |
Nippon Chemiphar Co., Ltd. (1st section of TSE, 4539) | Signed a contract for an exclusive license right for DFP-17729 in Japan. |
* The company signed a contract for an exclusive license right with Kyowa Chemical Industry Co., Ltd. (unlisted) for DFP-14323, in Japan, but consented to cancel the contract in November 2020.
(Taken from the reference material of the company)
1-4-3 Drug pipelines
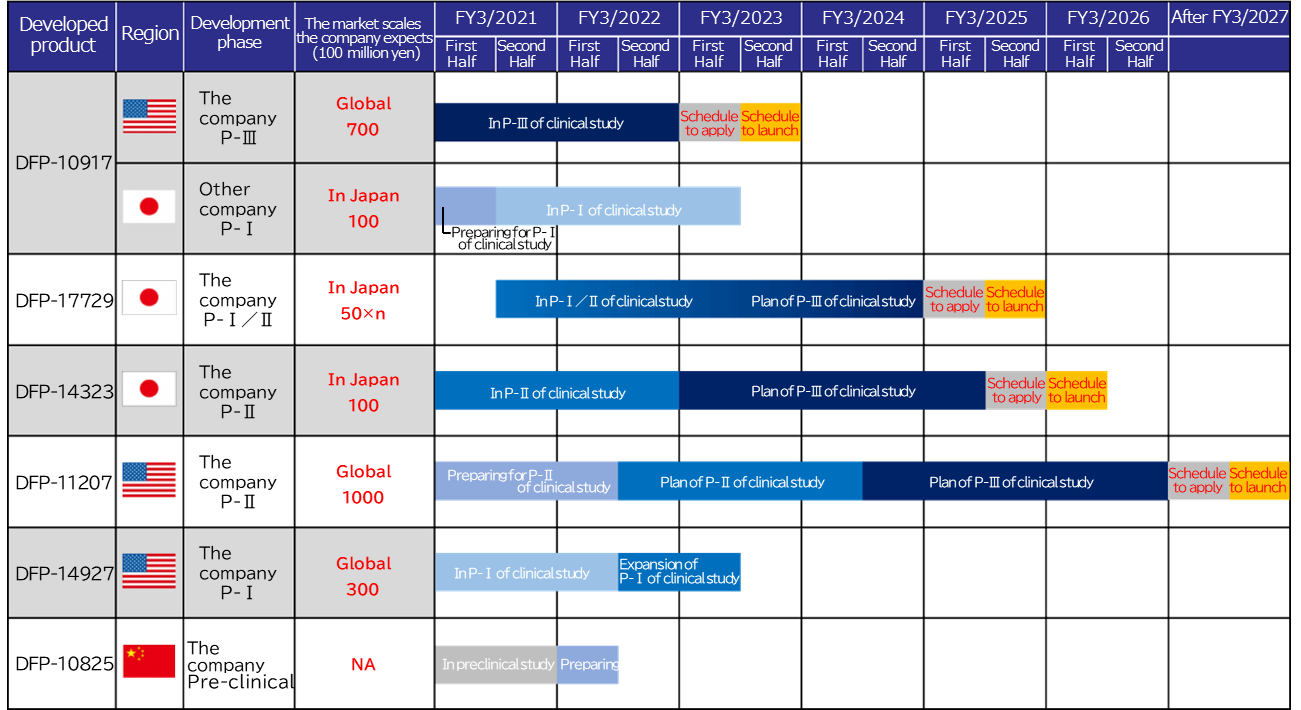
As of now, Delta-Fly Pharma has the following 6 drug pipelines in accordance with the above mentioned management policy.
The progress, current situation, and future plan for the development and commercialization of each pipeline are as shown below. Four candidate drugs are undergoing clinical studies. For the remaining two candidate drugs, clinical studies are being prepared.
(Taken from the reference material of the company)
1) 「DFP-10917」
Item | Outline |
Main target disease | Refractory and recurrent acute myeloid leukemia. The number of deaths associated with acute myeloid leukemia (AML) is 10 thousand in Japan, 30 thousand in the U.S., 30 thousand in Europe, and 20 thousand in China. 85% of the people who died from leukemia were 60 years old or older.
(Standard treatment methods have been established. About 70% of patients go into remission temporarily, as the cancer cells disappear from blood, but recurrence rate is high, and only 30% of patients can recover fully.) |
Characteristics of existing medicines, etc. | The existing medicine CNDAC is targeted at solid tumors. Dosage is high, and administration is conducted intravenously or orally in a short period of time. The efficacy against solid tumors is limited, and serious side effects were observed in some cases. |
Improved points and effects of modules | The dosage was reduced, and administration was conducted intravenously and continuously for a long period of time. As a result, there emerged different effects from those of conventionally used nucleic-acid derivatives (such as cytarabine and gemcitabine). It can be expected that the drug will be effective for the patients of refractory and recurrent acute myeloid leukemia, which cannot be treated with existing chemotherapy. The product excels in the balance between effectiveness and safety, and is optimal for the treatment of terminal hematologic cancer. |
Countries where patents were acquired (May 2021) | Japan, the U.S., EU, China, Australia, South Korea, and Russia |
(State of development, and future commercialization)
In the clinical phase I/II tests carried out in the U.S., the drug was effective for 48% (14/29) of patients in the phase II, indicating high effectiveness. Taking this result, the company had a meeting with the U.S. Food and Drug Administration (FDA) after the clinical phase II test, and submitted a plan for the clinical phase III test. Consent was obtained by US FDA, however, as the treatment guideline of refractory and recurrent acute myeloid leukemia was changed. After the startup meeting with US FDA, the company re-submitted the revised protocol for the clinical Phase III study and the screening of research subjects started.
In consideration of the impact of the new coronavirus infection, the number of hospitals participating in the clinical trial has been increased to 39 in order to promote case registration, and the Phase III clinical trial is underway.
As the company has already secured the active pharmaceutical ingredients and final preparations for the new drug application of DFP-10917, it aims to submit an application in the first half of the term ending March 2023 and launch the product in the second half of the term.
In May 2021, as part of the preparations for new drug application, DFP-19017 was given an international nonproprietary name of Radgocitabine by the committee on International Nonproprietary Names for Pharmaceutical Substances under the World Health Organization (WHO).
Deciding on an international nonproprietary name for a pharmaceutical substance is an important process of new drug approval, and the name so given will be used as a universal proper noun after the substance is approved as a new drug.
In Japan, Nippon Shinyaku Co., Ltd., the licensee, submitted a notification of a clinical study plan to the Pharmaceuticals and Medical Devices Agency (PMDA) on January 8, 2021 in order to initiate the clinical phase I study, and received permission from the PMDA on February 8 to conduct the clinical phase I study in Japan targeting refractory or relapsed AML patients.
Regarding the rights in territories outside Japan, negotiations for licensing contracts with pharmaceutical companies in Europe, the U.S., and China are in progress.
(Patent-related)
The company has applied for a patent globally in major countries for its invention of using DFP-10917 with the new derivative of Venetoclax (VTX) in combination treatment and as combination medication, and decision on granting a patent was made in Japan in June of 2021.
The new derivative of Venetoclax, for which the company submitted a patent, is a new substance acquired by forming a covalent bond between Venetoclax and a water-soluble polymer, and can selectively transport the active substance of Venetoclax to the targeted cancer cells; in experiments on animals transplanted subcutaneously human acute myeloid leukemia cells, it indicated similar results to the existing Venetoclax with less than a few tenths of the dosage while being safer.
The company intends to carry out clinical studies on combination treatment using DFP-10917 in combination with VTX with the aim of maximizing the markets and prolonging the exclusive marketing period in major countries following manufacturing and sale approval in the U.S. for use of DFP-10917 alone as slated for the term ending March 2023.
2) 「DFP-14323」
Item | Outline |
Main target disease | Terminal stage lung cancer, etc. |
Characteristics of existing medicines, etc. | The existing medicine “Ubenimex” (UBX) is targeted at blood cancer. The dosage is high, and administration is conducted intravenously or orally with a single agent. It is indicated that the drug is for blood cancer only, but it showed a survival advantage against lung cancer. |
Improved points and effects of modules | For the purpose of enhancing the antitumor effect, the dosage was reduced, and the drug was used together with a molecular target drug. As a result, the efficacy against lung cancer was confirmed. The immune function in cancer patients is improved, and the effectiveness of existing drugs is enhanced. The drug is expected to treat terminal or elderly patients of solid tumors. |
Countries where patents were acquired (May 2021) | Japan, the U.S., EU, Australia, Korea, Russia, Republic of China |
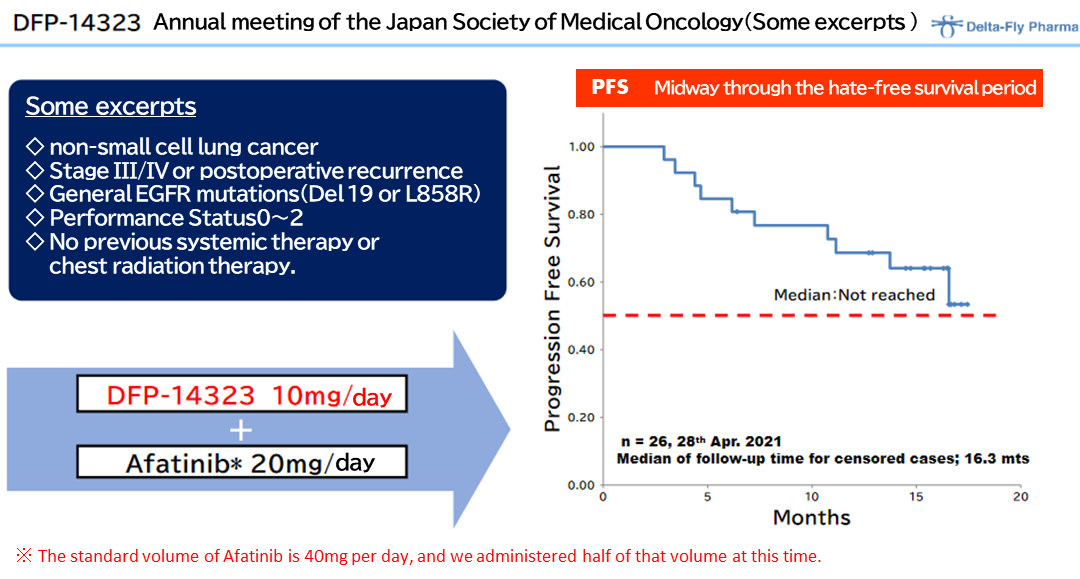
◎The clinical efficacy of DFP-14323
(Taken from the reference material of the company)
(State of development, and future commercialization)
As for the existing medicine Ubenimex, Nippon Kayaku Co., Ltd. obtained the approval for its efficacy and effect of “prolonging the survival period of adults suffering from acute non-lymphatic leukemia when combined with maintenance and intensive chemotherapeutic agents after remission” in Japan.
Delta-Fly started the clinical phase II study for the combination therapy of low-dose EGFR-TKI targeted at patients of EGFR gene mutation-positive non-small cell lung cancer as an additional indication in January 2018 in Japan and since the facilities that conduct the clinical studies have increased in Japan, the company proceeded with registering the new patients’ enrollment. In March 2020, the registration was complete for all patients’ enrollment.
DFP-14323 is the development code for obtaining approval as a Ubenimex’s new drug with expanded new indications.
Afterwards, during the effect measurement of the clinical phase II study based on the disease control rates (DCR) of all registered cases (including brain metastasis cases), a DCR of 100% was confirmed in June 2020, and efficacy was confirmed with the DCR of 100% and an overall response rate (ORR) of 65.4% or higher, in an effect measurement evaluation by independent medical doctors. The company thinks the product displayed excellent curative effects for brain metastases in patients of non-small cell lung cancer.
Further, based on the fact that it was discovered to be useful as a combination medicine for the treatment of patients of terminal non-small cell lung cancer with brain metastasis, the company made an international patent application to the member nations of the Patent Cooperation Treaty (PCT).
In addition, the results of the clinical phase II study conducted in Japan were presented in a poster presentation at European Society for Medical Oncology (ESMO) ASIA CONGRESS 2020 held in November 2020, showing the latest data on great safety and progression-free survival (PFS) confirmed in the study.
The data on PFS is important information for determining a protocol of the clinical phase III study, and the company plans to accelerate the clinical phase III study, which is scheduled to be started in the term ending March 2023, in cooperation with Japanese and overseas pharmaceutical companies that are highly interested in the clinical study data of the clinical phase II study.
Based on the favorable results of the clinical phase II study held in Japan as well as its intellectual property foundation, the subjects for the clinical phase III comparative study for DFP-14323 are scheduled to be Non-small cell lung cancer patients with brain metastasis, and by including China, which is said to have the largest number of lung cancer patients in the world, the company is proceeding with preparations to obtain approval and release DFP-14323 as soon as possible.
At the moment, the company plans to submit an application in the second half of the term ending March 2025 and launch the drug in the first half of the term ending March 2026.
The company signed a contract for an exclusive license right in Japan with Kyowa Chemical Industry Co., Ltd. (unlisted), but in November 2020, Kyowa Chemical Industry Co., Ltd. withdrew its plan for joint development and terminated the contract because of internal circumstances at Kyowa Chemical Industry.
The company will continue pursuing the manufacture and sales approval of the generic drugs of Ubenimex from PMDA on their own. Furthermore, the company will wait till around June 2021, for the evaluation of Progression Free Survival (PFS) and Overall Survival (OS), and apply to the PMDA for the manufacture and sales approval for the additional indication product for Ubenimex.
(Patent-related)
In May 2020, a patent was granted in Europe.
Currently, they have a pending patent application for DFP-14323 in the People's Republic of China and are following up on the evaluation process with the China National Intellectual Property Administration. When the patent is granted in the People's Republic of China, the company will have a foundation for expanding its business globally to major countries.
3) 「DFP-11207」
Item | Outline |
Main target disease | Solid tumors (such as pancreatic cancer) |
Characteristics of existing medicines, etc. | The existing medicine TS-1 has hematotoxicity, including the reduction of blood platelets, and it is difficult to continue treatment sufficiently. |
Improved points and effects of modules | DFP-11207 is a compound developed by combining three modularized active substances (modules I, II, and III) for sustained release, inhibition, and deactivation, in order to control the pharmacokinetics of 5-fluorouracil (5-FU), which has anticancer effects. It avoids hematotoxicity, including the decrease of blood platelets, which is caused by conventional 5-FU anticancer drugs, improves the balance between efficacy and safety, and enables long-time continuous treatment. This is a representative case of module drug development, in which the combination of compounds was improved. Optimal for preventing post-operation relapse or metastasis of micro cancer, and high life-prolongation effect can be expected. |
Countries where patents were acquired (May 2021) | Japan, the U.S., EU, China, Australia, Korea, Russia, Republic of China, Hong Kong |
(State of development, and future commercialization)
In the U.S., the company proceeded with the clinical phase I study for solid tumors (digestive system cancer), and determined the recommended dose at the next test and confirmed that the decrease of blood platelets does not occur as a side effect, which has been caused by conventional 5-FU anticancer drugs.
Currently, the preparations are progressing as testing the effects of food has finished, and the company summarized the process, held a discussion with the clinical investigators, and formulated the plan for the clinical phase II study with the combined use of anticancer drugs.
The company announced the results of the clinical phase I study and the food effects’ study at the conferences of the Chinese Society of Clinical Oncology (CSCO) and Japan Society of Clinical Oncology (JSCO) in 2019.
Moreover, in May 2020, the result of the clinical Phase I study in the US was published in the American cancer treatment journal “Investigational New Drugs.” The drug’s safety was confirmed as it does not cause diarrhea or platelet toxicity, does not require a withdrawal period, and leukopenia is mild, and it was recognized as a drug that could have a life-extending effect.
The company is negotiating with Chinese pharmaceutical companies interested in these American clinical data to open up opportunities for joint development between the U.S. and China.
At the moment, the company plans to submit an application in the first half of the term ending March 2027 and launch the drug in the second half of the term.
(Patent-related)
The company filed a patent to the member nations of the Patent Cooperation Treaty (PCT) and Taiwan after successful development of the stable preparation as a result of its concentrated efforts to improve the techniques of preparing DFP-11207 that is unstable and sensitive to humidity. A patent for the stable preparation, if granted, will provide a longer-term foundation for the intellectual property of DFP-11207.
4) 「DFP-14927」
Item | Outline |
Main target disease | Pancreatic cancer, gastric cancer, and myelodysplastic syndromes |
Characteristics of existing medicines, etc. | The existing medicine DFP-10917 needs to be administered for 14 days in a row, by using a pouch for continuous intravenous injection, and it was necessary to improve its convenience. The target disease has been only blood cancer. |
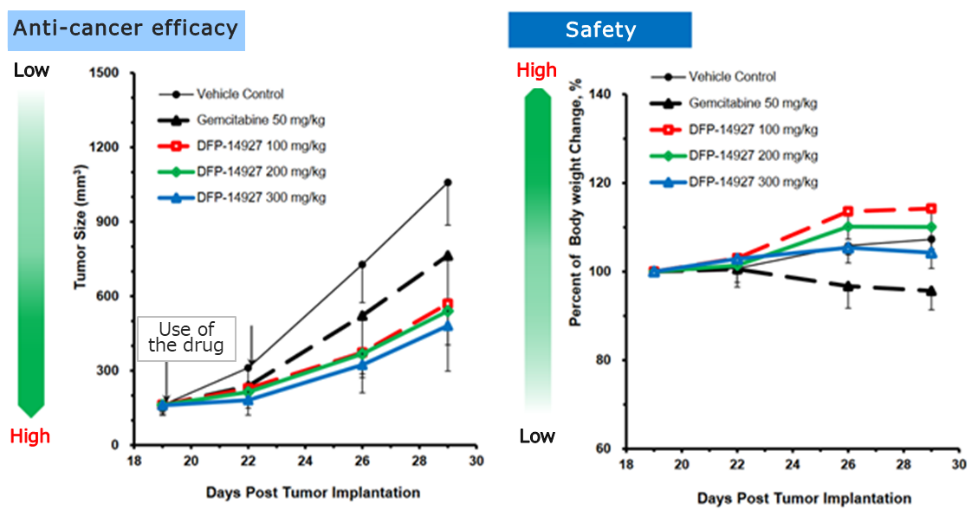
Improved points and effects of modules | DFP-14927, a polyethylene glycol-conjugated candidate anticancer substance, is a polymeric delivery of DFP-10917. It selectively clusters around cancer tissue, and discharges DFP-10917 effectively inside cancer cells. The frequency of administration was reduced to once per week, and intravenous drip infusion was adopted. As a result, the medicine now can be used against solid tumors and myelodysplastic syndrome as well as blood cancer. Additionally, in animal models with pancreatic cancer, it was confirmed to be more effective and safer than gemcitabine, the standard chemotherapy for pancreatic cancer. |
Countries where patents were acquired (as of the end of May 2021) | Japan, the U.S., China, Australia, Russia, Hong Kong |
◎Confirming DFP-14927 efficacy on animals
In animal models of pancreatic cancer, both the efficacy and safety of DFP-14927 were superior than gemcitabine, the standard chemotherapy for pancreatic cancer.
(Taken from the reference material of the company)
(State of development, and future commercialization)
The preclinical study has been completed in the U.S. The data of the preclinical study indicates that the level of the medicine in blood is stable for a long period of time when it is administered once a week, and that there is the antitumor effect against solid tumors.
In March 2018, the company concluded a contract for collaborative development with Sanyo Chemical Industries, Ltd. and prepared for the application for the start of the clinical phase I study, and on January 18, 2019, the U.S. FDA completed the examination of the safety of Investigational New Drug (IND), and approved the clinical phase I study in the U.S. And the company started clinical phase I study aimed at patients with digestive system cancer including pancreatic cancer and gastric cancer.
Due to the impact of the spread of the novel coronavirus, the case registration has slowed down in areas with large numbers of infected patients, but once the safety around the present dosage is confirmed, the company plans to select the optimal cancer, add multiple major cancer centers in the U.S., and move on to an extended study equivalent to the clinical phase II study in the second half of the term ending March 2022.
It also plans to discuss the possibility of the clinical phase I/II studies on myelodysplastic syndrome (MDS), which are a type of hematologic cancer.
Regarding distribution rights in territories outside Japan, negotiations for licensing contracts with pharmaceutical companies in Europe, the U.S., and China are in progress.
5) 「DFP-10825」
Item | Outline |
Main target disease | Gastric cancer, ovarian cancer, and peritoneal metastasis from pancreatic cancer |
Characteristics of existing medicines, etc. | Although the basic drug siRNA has a definite inhibitory effect as its basic effect, its clinical effect in systemic administration has been poor. |
Improved points and effects of modules | Nucleic acid drugs using RNA interference are expected to be the next cancer treatment drugs next to molecular-targeted cancer drugs and cancer immunotherapeutic drugs. The nucleic acid drug DFP-10825 is designed to be effective by intraperitoneal rather than systemic administration, as it specifically inhibits the factors that significantly affect cancer growth by RNA interference. In patients with ovarian cancer or stomach cancer, fluid retention such as pleural fluid and ascites (peritoneal metastasis) is observed at the terminal stage, but ascites is controlled by injecting the drug directly into the abdominal cavity to exert an effect. It is expected to relieve the pain and lead to the patients’ prolonging life. |
Countries where patents were acquired (Nov. 2020) | Japan, the U.S., EU, China, Australia, Korea, Russia, Republic of China, Hong Kong |
(State of development, and future commercialization)
The company has already completed efficacy and pharmacokinetics studies against peritoneal metastasis that causes ascites associated with ovarian, stomach or pancreatic cancer. Preliminary investigations based on the current Good Manufacturing Practice (cGMP) standards have also been completed for the manufacture of the clinical study drugs, such as drug substances, DDS and preparations. From now on, after adding preclinical studies according to the Good Laboratory Practice (GLP) standards for conducting non-clinical studies concerning safety of drugs using a part of the funds obtained from the stock listing, the company is planning to apply for IND to the US FDA and will begin the clinical phase I study for peritoneal metastasis of ovarian, stomach or pancreatic cancer patients in the U.S. The company has already received each country’s patent certifications.
The company will begin preparations for clinical studies in the first half of the term ending March 2022 as it is currently performing a preclinical study using animals as well as preparing active pharmaceutical ingredients and investigational products.
6) 「DFP-17729」
Item | Outline |
Main target disease | Terminal stage pancreatic cancer, malignant gastric lymphoma, gastric cancer, and lung cancer. |
Characteristics of existing medicines, etc. | Urinary alkalinizing agents, which are existing drugs, are targeted for hyperuricemia and others, but it has been confirmed that they provide a life-prolonging effect in pancreatic cancer and have an antitumor effect on each cancer tumor. |
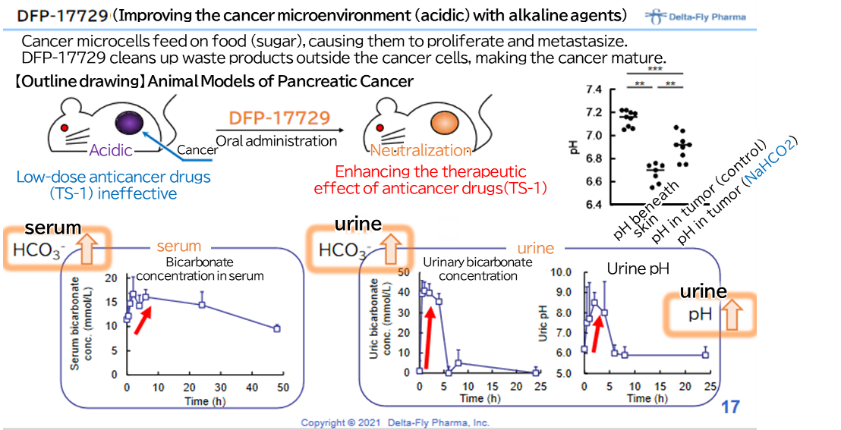
Improved points and effects of modules | Normal cells are more alkaline outside the cells than inside the cells, but cancer cells are more acidic outside the cells. This is because the growth of cancer cells promotes glycolysis, producing lactic acid and hydrogen ions, and they are actively released into the extracellular space. DFP-17729 suppresses the growth of cancer by alkalizing the outside of cancer cells. In other words, it cleans the area surrounding the cancer, and calms the cancer down. It has been confirmed in animal experiments that the combined use of an anticancer drug and an immune checkpoint inhibitor enhances the effect as compared with the monotherapy with an immune checkpoint inhibitor. |
Countries where patents were acquired (as of the end of May 2021) | Japan, Korea, Republic of China |
◎Confirming the clinical efficacy of DFP-17729
 |
 |
(Taken from the reference material of the company)
(State of development, and future commercialization)
The company is preparing for the additional indication of urine alkalizing agents, which are approved and sold as pharmaceutical products, as anti-cancer drugs in Japan.
Because urine alkalizing agents are already being used in clinical practices for the efficacy and effect of “acidosis improvement” to treat “hyperuricemia” and “tumor lysis syndrome,” so the preclinical study is not necessary.
The company aims to expand the range of anti-tumor effects of existing drugs through the combined use of anti-cancer agents and an immune checkpoint inhibitor and provide new cancer treatments.
In March 2020, the company signed a licensing contract with Nippon Chemiphar Co., Ltd., by which it agreed to give Nippon Chemiphar Co., Ltd., the exclusive marketing right and the exclusive manufacturing right of DFP-17729 in Japan.
Delta-Fly Pharma will perform clinical studies of the combined use with existing anti-cancer agents for pancreatic cancer patients, while Nippon Chemiphar Co., Ltd. will be responsible for manufacturing and selling DFP-17729 in Japan after the PMDA approval.
In May 2020, the company submitted a paper about DFP-17729 and it’s accepted by the journal of American Association for Cancer Research “Molecular Cancer Therapeutics.”
Generally, the 5-year survival rate of pancreatic cancer patients is less than 10%, which is severely low. However, this research indicates that it does not only increase the efficacy of existing pancreatic cancer treatments, but also increase the effectiveness of an immune checkpoint inhibitor (anti-PD-1 antibody). Moreover, DFP-17729 does not show any of the side effects of the existing anti-cancer drugs and it was confirmed that it does not produce extra toxicity from combining it with existing anti-cancer drugs.
After obtaining these results, the company submitted a clinical trial plan to the PMDA in July 2020 with the aim of executing the clinical phase I/II studies in multiple medical institutions in Japan, targeting patients with terminal pancreatic cancer, and received permission for the execution of the studies after completion of an examination by PMDA.
Taking the condition of the terminal pancreatic cancer patients into account, the clinical study will be used to investigate and confirm the safety/effectiveness of the clinical phase I/II studies before transitioning into the clinical phase III study. The clinical phase I study will confirm the safety when using the existing drugs and DFP-17729 at the same time, and the clinical phase II study will be a comparative test to confirm whether DFP-17729 excels compared to existing drugs.
The clinical phase I study had been carried out since patients’ enrollment for the study began on November 18, 2020 at three major hospitals in the Kanto region. Following completion of the period of evaluating the safety for all of the patients enrolled in the study, the safety of DFP-17729 when used in combination with anticancer drugs was confirmed through deliberations on April 15, 2021 by the safety evaluation committee, and the company was permitted to move on to the clinical phase II study.
In the clinical phase II study in which six major hospitals participate, the drug was administered to the first patient on April 22, 2021.
As this study is performed as clinical phase I/II studies, the company made an almost seamless transition from the safety confirmation by the safety evaluation committee in the clinical phase I study to the start of administration of the drug in the clinical phase II study.
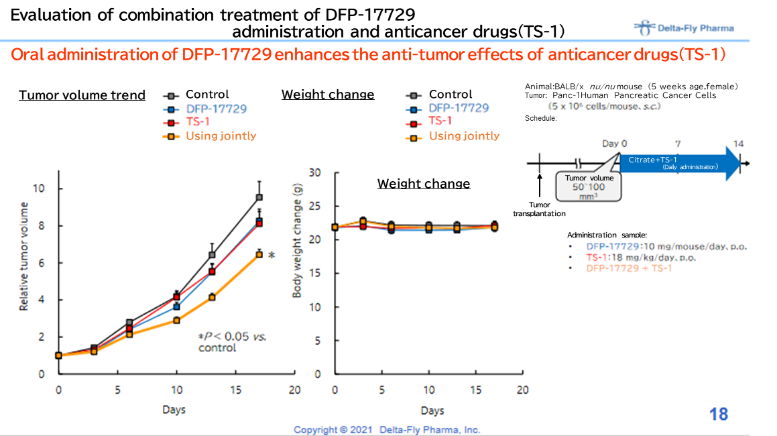
Meanwhile, in January 2021, the company announced that it was confirmed, using animals to which human pancreatic cancer cells were transplanted, that DFP-17729, an agent for improving the cancer microenvironment, boosts the therapeutic effect of TS-1 which is a drug against pancreatic cancer. The company believes that this discovery has provided firm foundations for development technology and intellectual property in Japan for taking indications for not only pancreatic cancer but also for other cancers, such as malignant melanoma, gastric cancer, and non-small cell lung cancer.
After proceeding with the clinical study in Japan in collaboration with the company’s partner, Nippon Chemiphar Co., Ltd., the company plans to expand into Europe, the U.S., and various Asian countries in the future, based on the clinical study data obtained in Japan.
At the moment, the company plans to move on to the clinical phase III study in the term ending March 2023, submit an application in the first half of the term ending March 2025, and launch the drug in the second half of the same term.
1-5 Four characteristics as a bio-venture
The company as a bio-venture has the following four main characteristics.
1) Module drug development
As described above, the company is patenting existing drugs, etc. by re-inventing them with ingenuity based on “modules” (components) and creating new drugs with improved balance between clinical efficacy and safety.
2) Specialized in the development of anti-cancer drugs
By working specifically on “anti-cancer drugs,” which still have limited effectiveness and cause various side effects, the company is accelerating the development of new drugs through module drug development and contributing to the improvement of the social life of cancer patients.
3) Development by experienced members
The development members consisting of people who have been engaged in research and development of anti-cancer drugs for many years at pharmaceutical companies and clinicians who are familiar with cancer patients advance the development of drugs with certainty and meet unmet medical needs. This sharply differentiates the company from others, giving competitive advantage.
4) Effective utilization of external resources
The company operates efficiently by focusing on management and operation of research and development without having factories or research institutes and proactively cooperating with external contractors and other organizations for outsourcing tasks.
2. Earnings Trends
2-1 Fiscal Year ended March 2021 Earnings Results
1) Earnings trends
| FY 3/20 | FY 3/21 | YoY |
Operating Revenue | 100 | 300 | +200 |
Operating Cost | 1,645 | 1,152 | -493 |
R&D Expense | 1,397 | 866 | -530 |
Other SG&A expenses | 248 | 285 | +37 |
Operating Income | -1,545 | -852 | +693 |
Ordinary Income | -1,552 | -859 | +692 |
Net Income | -1,555 | -862 | +693 |
Unit: Million yen
(Operating Revenue)
The company earned milestone income through the licensing agreements with Nippon Chemiphar Co., Ltd. and Nippon Shinyaku Co., Ltd.
(Operating Cost)
The number of medical institutions undertaking the clinical studies for pipelines under development and the number of patients’ enrollment increased, and progress was made in manufacturing of active pharmaceutical ingredients and preparations as investigational products for next studies.
(Operating income)
Operating loss shrank 693-million-yen year on year to 852 million yen.
2) Financial Conditions and Cash Flows
◎Main BS
| End of Mar. 2020 | End of Mar. 2021 |
| End of Mar. 2020 | End of Mar. 2021 |
Current Assets | 2,115 | 2,115 | Total Liabilities | 105 | 82 |
Cash | 1,943 | 2,088 | Total Net Assets | 2,056 | 2,078 |
Noncurrent Assets | 46 | 45 | Retained Earnings | -3,622 | -4,484 |
Property, Plant and Equipment | 43 | 41 | Total Liabilities, Net Assets | 2,162 | 2,161 |
Total Assets | 2,162 | 2,161 | Balance of Short and Long-Term Debts | 5 | - |
Unit: Million yen
Total assets stood at 2,161 million yen, almost unchanged from the end of the previous year owing to an increase in cash and a decline in accounts receivable.
The capital stock and reserve increased following the exercise of share options while retained earnings decreased, raising net assets by 22 million yen from the end of the previous year to 2,078 million yen.
The equity ratio grew 1.0 point from the end of the previous year to 96.1%.
3) Status of clinical development at the novel coronavirus
◎Phase II clinical trials for DFP-14323 (late-stage lung cancer) and Phase I/II clinical trials for DFP-17729 (late-stage pancreatic cancer)
Progressing well in Japan.
◎DFP-10917
In the U.S., the company is currently conducting Phase III comparative clinical trials. In light of the impact of the novel coronavirus, it continues to aim to obtain approval in the U.S. by the end of FY2022, by increasing the number of clinical trial sites.
In Japan, Nippon Shinyaku Co., Ltd., the licensee, submitted a notification of a clinical study plan to the Pharmaceuticals and Medical Devices Agency (PMDA) on January 8, 2021 in order to initiate the clinical phase I study, and received permission from the PMDA on February 8 to conduct the clinical phase I study in Japan targeting refractory or relapsed AML patients.
In addition, for the purpose of expanding DFP-10917 related business, the company is considering a combination study with Venetoclax (VTX), and is also working on the development of a PEG drug conjugate (PEGDC) that enables cancer-selective drug delivery of VTX. As the effectiveness of the drug has been confirmed in animal experiments and the substance patent has been granted in Japan, the intellectual property base for developing the drug in Japan as a candidate for the next new drug is now in place.
◎DFP-14927 (polyethylene glycol conjugated form of DFP-10917)
Antibody-drug conjugates (ADCs), in which anti-cancer substances are covalently bound to antibodies that target cancer cells, are gaining prominence as next-generation anti-cancer drugs. The company is developing DFP-14927 as PEGDC, and the substance patent has been granted in major countries in the world, including U.S, China, and Japan.
Under the guidance of the U.S. Food and Drug Administration (FDA), DFP-14927 is currently undergoing Phase I clinical trials at M.D. Anderson Cancer Center in the U.S. to confirm its safety and efficacy in patients with solid tumors, and is scheduled to enter expanded Phase II clinical trials in April 2021 at two additional U.S. cancer centers.
◎DFP-10825
Although there was a delay in arranging laboratory animals from Wuhan, China, safety testing has resumed in China as the corona infection in Wuhan has subsided. In addition, the manufacture of the nucleic acid material and drug transport carrier for the drug substance was completed in Japan, and the manufacturing of the investigational drug will start in March 2021 at a manufacturing company in the US. Four related inventions for DFP-10825 have been granted patents in the US, Europe, China, Japan, and other countries, and seven papers on its effectiveness have been published in top European and US journals.
In light of the impact of the novel coronavirus, the company plans to proceed with the clinical development of the next DFP-10825 drug candidate, which was to be conducted in the U.S., mainly in Japan, where the drug is safer.
2-2 Fiscal Year ending March 2022 Earnings Forecasts
| FY 3/21 | FY 3/22 (Estimate) | YoY |
Operating Revenue | 300 | 100 | -200 |
SG&A | 1,152 | 1,400 | +247 |
R&D Expenses | 866 | 1,090 | +223 |
Other SG&A Expenses | 285 | 310 | +24 |
Operating income | -852 | -1,300 | -447 |
Ordinary income | -859 | -1,300 | -440 |
Net loss | -862 | -1,300 | -437 |
Unit: Million yen
(Operating Revenue)
Operating revenue as milestone compensation for licensing agreements is estimated at 100 million yen, down 200 million yen year on year.
In addition to revenue from milestone compensation for DFP-10917, the company is expected to earn revenue, such as lump-sum contract payments through alliances with new partners, as the clinical studies of several candidate compounds for anticancer drugs, including DFP-10917 which is undergoing the clinical phase III study in the U.S. and DFP-14323 which is going through the clinical phase II study in Japan.
The company plans to announce its future outlook in a timely manner when revenue is finalized.
(Operating Cost)
Operating cost is projected to grow 250 million yen year on year to 1.4 billion yen.
R&D expense is expected to rise 223 million yen year on year so that the company can make steady progress with each of the pipelines under development.
(Operating loss)
Operating loss will stand at 1.3 billion yen, up 447 million yen year on year.
3. Growth Strategy
The company will firmly continue the development of the 4 products undergoing the clinical studies and the 2 products undergoing preparation for the clinical studies, and from FY 2022, it aims to steadily release them to the market. Further, the company plans to expand profitability and focus on securing alliance partners in Japan, China, Europe, and the U.S.
4. Conclusions
D-10917, which will be launched as the first product developed by the company, has been given an international nonproprietary name of Radgocitabine. Although the company estimates the maximum sales volume of DFP-10917, Radgocitabine, to be 80 billion yen (70 billion yen from the global market and 10 billion yen from the Japanese market), it considers that the scale of the acute myeloid leukemia (AML) market is even larger. We would like to keep an eye on information released by the company regarding the progress with the clinical phase III study, which is being carried out this fiscal year, before the company submits an application and launches Radgocitabine in the U.S. in the term ending March 2023 as planned.
<Reference: Regarding Corporate Governance>
◎Organization type, and the composition of directors and auditors
Organization type | Company with audit and supervisory board |
Directors | 8 directors, including 4 outside ones |
Auditors | 3 auditors, including 2 outside ones |
◎Corporate Governance Report
The latest update: July 1, 2020.
<Basic policy>
Our company thinks that our mission is to operate our business while putting importance on the benefits of all stakeholders, including shareholders, clients, business partners, employees, and local communities, under the mission of “To provide treatment methods recommendable for cancer patients and their families with peace of mind through module drug development.” To accomplish this, it is indispensable to develop our business stably and perpetually. Our basic policy for corporate governance is to improve systems for securing the soundness, transparency, and efficiency of business administration, which will become the base for the above-mentioned development.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code>
It is written that “We follow all of the basic principles.”
This report is intended solely for information purposes and is not intended as a solicitation for investment. The information and opinions contained within this report are made by our company based on data made publicly available, and the information within this report comes from sources that we judge to be reliable. However, we cannot wholly guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration. Copyright(C) Investment Bridge Co., Ltd. All Rights Reserved. |

