| Human Metabolome Technologies (6090) |
|
||||||||
Company |
Human Metabolome Technologies Inc. |
||
Code No. |
6090 |
||
Exchange |
Mothers of Tokyo Stock Exchange |
||
Industry |
Service Industry |
||
President |
Ryuji Kanno |
||
Address |
246-2 Mizukami, Kakuganji, Tsuruoka-city, Yamagata |
||
Year-end |
End of March |
||
URL |
|||
*Share price as of closing on the end of December 9. Number of shares outstanding is as of September 30.
ROE and BPS are based on actual results of the previous term end. |
||||||||||||||||||||||||
|
|
*Forecasts are those of the Company. From the current fiscal year, the definition for net income has been changed to net income attributable to parent company shareholders (Abbreviated as parent net income).
|
|
| Key Points |
 |
| Company Overview |
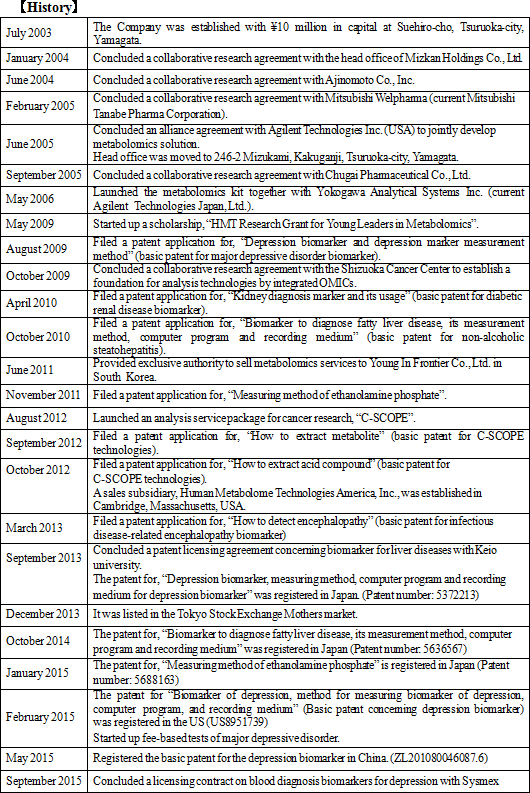 Metabolome analysis techniques had been used for various purposes such as fundamental biological research, development of pharmaceutical products, disease biomarkers, etc., and were expected to expand the demand in the future. Therefore, upon establishment of the CE-MS system, Human Metabolome Technologies Inc. was established in July 2003 to commercialize the method chiefly by Professor Soga, Professor Masaru Tomita, also from Keio University, and the office of Keio University. It was the first venture company originated from Keio University, with a financial support from Keio University's entrepreneurship fund. In 2008, Mr. Ryuji Kanno became the president of the Company. Before taking up this position, Mr. Kanno who was the Vice President & CEO of Agilent Technologies Japan, Ltd., a Japanese subsidiary of Agilent Technologies, a global company that develops, manufactures, sells, and supports chemical analysis equipments and electric & electronic measurement equipments in the field of life science., which, had been for sometime in business relationships with HMT and Keio University. President Kanno promoted research and development of the Company's core technologies and also began organizing and establishing more specific commercialization process and business models. At the same time, he began preparation for the Company to be listed on the stock market in order to accelerate its growth speed through enhancing the Company's visibility and raising funds for research and development. The Company got listed on the Mothers Section of the Tokyo Stock Exchange in December 2013, ten years after its establishment.  (1) Significance of social presence
Biomarkers are in vivo substances that are used as indicators to assess current state of specific diseases. Blood sugar" for diabetes, "γ-GPT" for liver function disorder, and "uric acid" for gout. are representative examples of well-known and widely used biomarkers.The Company discovered a biomarker for "major depressive disorder", one of current major social issues, and is developing diagnostic agents to quantify the condition of the disease. Because there is no prevailing method to objectively measure the status of depression although the number of patients of depression is increasing, some serious problems are emerging regarding to the therapy of depression. For example, patients who would have been cured if they had properly treated are still suffering or in overprescription. If the diagnostic agents using the Company's biomarker become to be widely used, these issues are expected to be solved, and social loss will be subsequently reduced.This social significance cannot be overlooked to understand the Company. (2) Excellent technological capacities
The Company is highly recognized thanks to the "metabolome analysis technology" which is used to examine complicated behavior of metabolites in human bodies to identify biomarkers. The biomarker for major depressive disorder is just an example. With the technology, the Company is expected to identify and develop various new biomarkers in the future. (3) Stable business model
The Company's current core business is the "commissioned metabolome analysis business", supporting research and development activities of research institutions and pharmaceutical companies, that occupies about 80% of its sales. Sales of FY March 2015 were 566 million (up 9.7%, YoY) and operating income was 313 million (up 0.9%, YoY), showing steady income. On the other hand, the "biomarker business", which is expected to achieve significant growth in the mid- to long-term, is still operated in a small scale and experiencing losses. However, the Company has already established a balanced business model, in which the profit generated from the commissioned metabolome analysis business is invested into the biomarker business for its growth. This model is notable among many bio-venture companies that are suffering from gaining profit. ◎What is depression?
Depression is a type of mood disorders, and is a brain dysfunctional state for various reasons such as accumulated physical and mental stress. Because the brain is not functioning properly, people in the depressed mood feel negative and low-esteem. This causes vicious cycle in which people with depression feel more stress for matters that they could otherwise handle. The "major depressive disorder" refers to the state in which depression mood continues even after the sources of stress are removed. In this regard, it is distinguished from adjustment disorders or some anxiety disorders, and is considered a brain dysfunction, instead of a mere response to stress. (Note: "Major" of the major depressive disorder means "primary" and does not mean "serious" depression.) ◎Number of depression patients in Japan
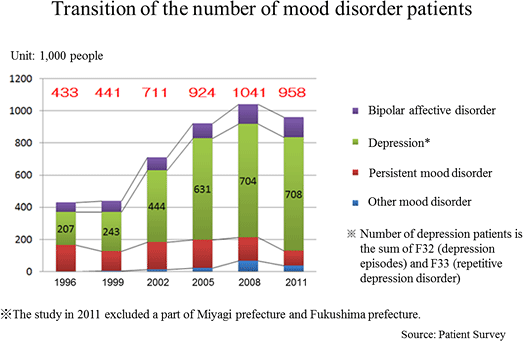
According to the "Patient Survey" conducted by the Ministry of Health, Labor, and Welfare (MHLW) every three years targeting health facilities across the country, the total number of patients with mood disorders including depression increased from 430,000 in 1996 to 950,000 in 2011 (2.2x). The "Patient Survey" shows statistical data of the number of patients who visit health facilities. It is known that the consultation rate of patients with depression is low. Thus, according to the MHLW, it is suspected that the actual number of depression patients might be larger.
 ◎Treatment of depression
If someone is diagnosed as having depression, the common treatment is prescription of "antidepressant drugs." The antidepressant drugs can be categorized into several groups such as selective serotonin reuptake inhibitor (SSRI), serotonin-noradrenaline reuptake inhibitor (SNRI), and tricyclic antidepressant. In addition, anti-anxiety drugs or sleep inducing drugs can be used, depending on the symptom. The important thing under drug therapy is that the patients comply with the prescribed amount and frequency after they are informed the effects and side effects. However, patients with depression often reduce the amount or frequency without doctors' permission as they do not feel the symptom to be very serious, they worry side effects, etc. In these cases, the patients do not show improvement as doctors expect, and the doctors prescribe more drugs or change the types of drugs. That would often result delay in recovery or excessive administration of drugs, for lack of trust between doctors and patients. Therefore, it is essential to have objective assessment standards quantifying the state of depression or proving the depression has been cured. The biomarker and diagnostic agents of the major depressive disorder that the Company is currently working on are extremely important for prompt and appropriate treatment of the disease. <What is metabolomics?>
Living organisms including human beings consist of some parts with various functions such as muscles, internal organs, and bones "Metabolites" such as amino acids, fatty acids, and nucleic acids are common and major components of these organs. Metabolites play crucial roles for entire life activities. Metabolites are provided by food, and are consumed in the process of daily actions such as exercise. They move in a body and cells in accordance with their functions, and are converted into new substances through various chemical reactions, which are called "metabolism". Adjusting body temperature, breathing, moving heart, digesting and absorbing food, transforming old cells into new ones are all operated by metabolism. The "substance conversion" to a new substance is based on a certain rule flow called metabolic pathway. One of well-known approaches to understand mechanisms of a human body is "genomics", analyzing genes. Now, automated sequencing and computer analysis of genetic information (DNA base sequences) is available, and nearly all the information in the human genome has already been deciphered. However, much about the relationship between the roles of genes and diseases remains unknown. Recently, more researchers lean towards investigating metabolic profiles, in addition to genetic information coming out of genome analysis, in order to understand the relationships between a human body and diseases. Consequently, research and use of "metabolomics (metabolome analysis)" targeting all metabolites is becoming increasingly popular.  <What are biomarkers?>
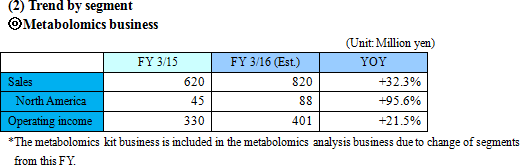
A human body puts various vital functions under high-sophisticated manipulation to minimize internal and external influences, and subsequently keeps the body condition stable. That mechanism is called "homeostasis". For example, body temperature and heartbeat may change temporarily, but return to regular t ranges. Diseases lead abnormal homeostasis and metabolic status, which are different from healthy conditions. A metabolite, which concentration keenly reflects status of a specific disease, is called a biomarker. By measuring a biomarker, the current status of a specific disease can be objectively assessed. Blood sugar as a pancreas function indicator, γ-GTP as a liver function indicator, biomarker PSA for prostatic cancer and biomarker CA19-9 for pancreatic cancer. are examples of well-known biomarkers. Biomarkers have been studied for a long time in order to monitor the status of diseases. These days, with new methods to analyze multiple substances with higher sensitivity all at once, study results of various new biomarkers have been publishing one after another. Among the biomarkers that are explored through metabolomics technologies are the followings:  Meanwhile, the Company is investing the income gained from its currently major business, "commissioned metabolome analysis business", into research and development of the growing business, "biomarker business". It is aiming for mid- to long-term growth through practical application of intellectual properties obtained from the biomarker business in the fields of pharmaceutical products development and disease diagnosis. The profit making structure and customers of each business are as follows. 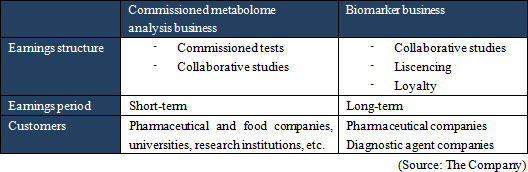 (1) Commissioned metabolome analysis business
The Company's customers are private companies such as pharmaceutical companies and food companies as well as universities and public research institutions, which order the commissioned metabolome analysis. The scheme of the service is as follows. A costumer send samples to the Company, and then metabolites are extracted from the samples. The extracted metabolites are measured by CE-MS system, and the acquired data is analyzed. After all, the report is delivered to the customer. The data obtained from the service are used for various purposes; basic biological study, assessment of drug effectiveness and toxicity assessment by pharmaceutical companies, universities, and research institutions, analysis of fermentation process and functional evaluation of functional foods by food companies. The data is contributing to the progress of research and development activities of customers.
"Sales were ¥566 million and operating income was ¥313 million for FY March 2015." ◎Deployment in the overseas market
In order to offer the commissioned metabolome analysis service in Asia, the Company concluded an exclusive sales authority agreement with Young In Frontier Co., Ltd. in South Korea in June 2011. Furthermore, in order to expand its business in the North American market, it also established its sales subsidiary, Human Metabolome Technologies America, Inc., in October 2012 at Cambridge, Massachusetts, USA, home to many medical research institutions. Its core product is C-SCOPE, a service package of the commissioned metabolome analysis service for cancer study. As a part of sales promotion activities, C-SCOPE has been provided for free or at discounted prices to famous cancer researchers in major universities to obtain high recognition to the technology, and then in order to penetrate into the North American market as soon as possible.
In August 2012, the Company launched "C-SCOPE", a service package of the commissioned metabolome analysis service well organized for cancer study. C-SCOPE was developed to respond to the needs that they would like to measure specific metabolites which are changing within cancer cells with higher sensitivity and higher accuracy. Its technologies are based on unique and efficient metabolites extraction method from cancer cells and highly sensitive analytical system. Cancer is the number one cause of death in Japan since 1981 and occupies about 30% of the recent total causes of death. According to the MHLW, the costs for cancer research are increasing year by year; in 2012, ¥35.7 billion were spent. It is an urgent task for many pharmaceutical companies to develop effective new anticancer drugs. The "Warburg effect" is the phenomenon that most cancer cells have glycolytic rates of several to dozens of times to that of normal cells. Although this effect was proposed as far back as over 80 years ago, the research on the effect made little progress since there was no method to comprehensively measure metabolites back then. Thanks to the dramatic advancement in metabolomics technologies in recent years, development of anticancer drugs which act as metabolic inhibitors are underway. The Company's metabolome analysis technology based on the CE-MS system is considered as one of the effective analysis systems applicable at each stage of cancer research; from basic study of cancer biology to clinical application in the process of anticancer drugs development. (2) Biomarker business
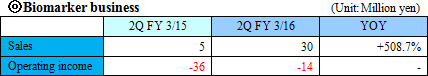
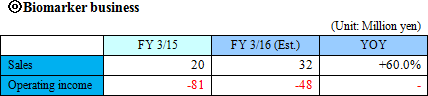
The Company considers the business related to biomarkers, which play an important role in occasions such as early diagnosis or monitoring treatment effects, as a driver for future growth, and is proceeding with biomarker discovery and development of clinical test drugs through collaborative research and development with universities, pharmaceutical companies, and diagnostic drugs companies. "Sales were ¥20 million and operating loss was ¥81 million for FY March 2015." The sales in this business come from: a) development of biomarker diagnostic methods and their commercialization as in vivo diagnostic drugs in collaboration with diagnostic drugs companies, b) cooperation money for research and development by introducing the in vivo diagnostic drugs in the clinical tests for developing new drugs or expanding application of existing drugs by pharmaceutical companies, or c) loyalty gained from the sales of the drugs when they are commercialized. ◎ Intellectual property policy
The personnel in charge of intellectual property and contracts work on patent application and request for examination of all projects, and are in close collaboration with the patent attorneys of the Company and its collaborative research institution. They are also responsible for negotiation concerning collaboration research agreements and development of agreement documents. The Company attempts to maximize its rights when obtaining a patent of newly discovered biomarkers. Since the scope of the rights differs depending on the marker, the Company generates patent application documents in a way that would cover wide scope of the rights such as chemical structure of the biomarker, usage for diagnostics and drug development, detection method and measurement equipment. Furthermore, the Company makes it a principle to file international patent applications in accordance with the Patent Cooperation Treaty, in anticipation for the future license agreement and market based on the information of clinical test drugs, test equipment companies and pharmaceutical companies of various countries. ◎Example of biomarker business: Major depressive disorder biomarker
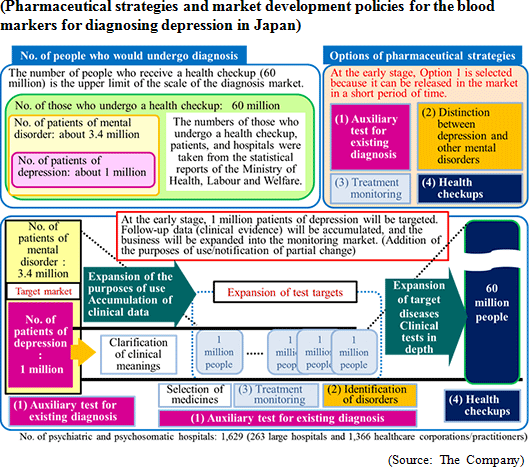
The Company especially focuses its research and development on: a) the central nervous system disorder such as depression (e.g. mood disorder, and mental disorder) for which there are few objective diagnosis methods and b) diseases that have become a social problems such as metabolic syndrome, including hepatitis and diabetes, and their related diseases. Its current focus is the biomarker for "major depressive disorder". Diagnosis of major depressive disorder is conducted using the diagnostic standards provided by the American Psychiatric Association or the standards of the World Health Organization (WHO). However, both of them largely reflect the subjective view of the doctor or patient, and unlike other diseases, no diagnostic method exists that has been established on the basis of objective indicators. The Company conducted collaborative research with the National Center of Neurology and Psychiatry, and discovered a blood biomarker of major depressive disorder. Blood samples were collected from approximately 30 patients and 30 healthy people, and the blood components were compared through metabolome analysis using the CE-MS system. As a result, the patients with major depressive disorder showed lower concentration of phosphoric ethanolamine (PEA) in their serum. Through further analysis, it was found that a) PEA is a specific biomarker for major depressive disorder, and b) PEA level will return to the healthy standard range when MDD is treated. 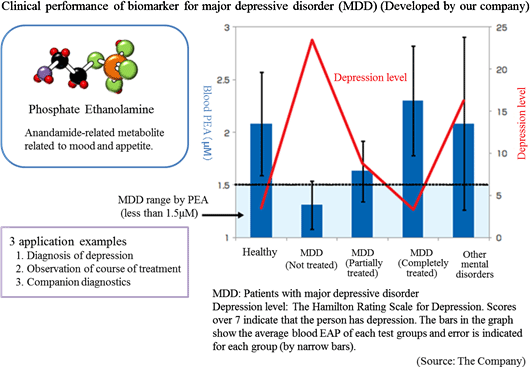 *Companion diagnostics: Refers to clinical tests to predict effects and side effects of pharmaceutical products before administration. By checking the responses of individual patient towards a drug before treatment, more effective drug administration can be provided.
◎Identification of disease biomarkers
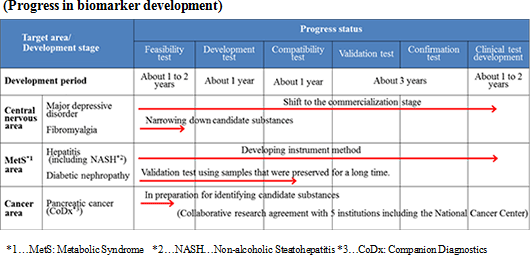
The Company uses the following three connections and systems for identifying biomarkers in order to expand biomarker development pipelines.
<Connection with the customers for commissioned analysis or collaborative development>
The Company accepts requests from universities and companies for the tests for finding biomarkers. The Company also receives proposals for collaborative development before or after the tests. Currently, a collaborative development project for diabetic nephropathy biomarkers is ongoing.
<Direct proposal to researchers and physicians>
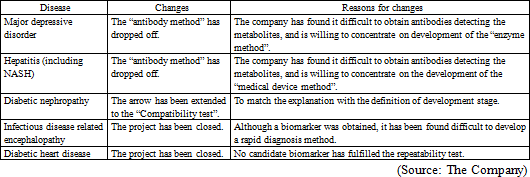
The Company's researchers directly propose research plans for the development of disease biomarkers to researchers or physicians, and establish collaborative study agreement with the institution based on approvals from the collaborative researchers or physicians. The target diseases are chosen according to the number of patients, compatibility to the analysis technologies of the Company, degree of social contribution, and necessity of biomarkers. In addition to major depressive disorder, the Company is working on the development of biomarkers for non-alcoholic steatohepatitis and fibromyalgia.
<HMT Research Grant for Young Leaders in Metabolomics>
The Company offers a grant (HMT Research Grant for Young Leaders in Metabolomics) to graduate students to disseminate the usefulness of metabolomics in the society and nurture young researchers. From the research themes submitted from graduate students across the world, the Company chooses excellent proposals, and supports their research by awarding metabolome analysis service without a fee. Fourteen students have been awarded in the last 4 years. Some of these study results actually led to identification of biomarkers and evolved to collaborative study with the Company, for example, infectious disease-related encephalopathy biomarker.
  |
| First half of Fiscal Year March 2016 Earnings Results |
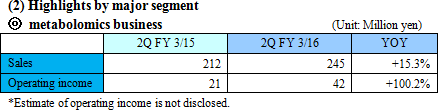
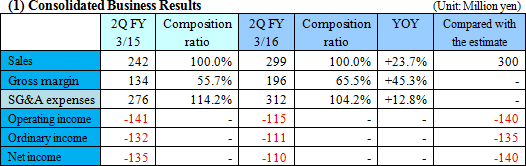 Sales increased, but there remained a loss due to the increase in investment for growth.
Sales were 299 million yen, up 23.7% year on year. The metabolome analysis business remained healthy both inside and outside of Japan. SG&A expenses increased as more sales staff and biomarker business staff were employed for growth, and subsequently personnel cost and investment in R&D augmented by 3.1 million yen and 8 million yen, respectively. As a result, an operating loss was sustained. Sales were nearly equal to the estimated value,
  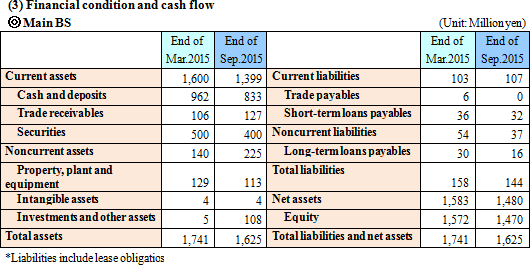 As the result, equity ratio increased from 90.9% at the end of the previous term to 91.1%. 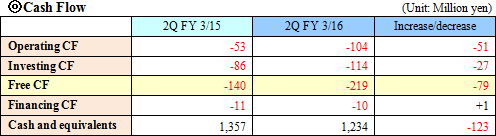 |
| Fiscal Year March 2016 Earnings Estimates |
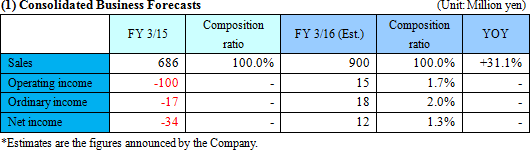 No revisions to the earnings forecast. The company aims to move into the black by boosting sales.
The earnings forecast has not been changed from the previous announcement. The estimated full year sales is 900 million yen, up 30% year on year. It is expected that large scale metabolome analysis projects will be ordered in the following half. The company will continue investments for growth, such as recruitment and R&D. The company is planning to turn to profit due to growth of sales and profit in the metabolome analysis and reduction of loss in the biomarker business.
 External environment looks good
In association with enforcement of the new Food Functionality Labeling System (*), it is expected that demand for metabolome analysis by companies which need analysis reports will increase. There is also a budget increase in research areas concerning diet, health, and preventive medicine which lead to good health and longevity. Furthermore, the Japan Agency for Medical Research and Development (*) was founded, and the systems to support new drug creation and promote development of innovative drugs are being established (*) In addition, the amount of budgets in cancer research in the US is approximately 18 times larger than that of Japan, and room for market development for the Company's analysis service for cancer research, "C-SCOPE", is very large. Thus, the external market environment for the business is good.
*New Food Functionality Labeling System
The System was established in June 2013 as a part of the deregulation measures in the Japan Revitalization Strategy. The purposes are "to provide appropriate information for the public to protect their own health, and to establish frameworks promoting simpler labeling rules of food functionalities than those in foreign countries for overseas expansion of Japanese agricultural products". This category was established following the existing categories, Food for Specified Health Uses (FOSHU), and Food with Nutrient Function Claims (FNFC). Products meeting certain standards regarding to such as safety and functionality are allowed to display "its health efficacies" and "its functionality" on the company's or producer's own responsibility. In order to acquire authorization to the FOSHU label, the products need to go through clinical tests, which are burden to the companies and producers in terms of cost and time. On the other hand, the new System requires only to submit a systematic review of the food or functionality-related components. *Japan Agency for Medical Research and Development Based on the "Plan for Promotion of Medical Research and Development", the Japan Agency for Medical Research and Development was launched to accelerate application of the basic research results in the arena of drugs and medical equipment with a focus on nine fields including regenerative medicine, cancer, cognitive impairment, etc., and to achieve the world's highest standard of treatment technologies. The Agency manages more than ¥140 billion funded by the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labor and Welfare, and the Ministry of Economy, Trade and Industry, and distributes the funds strategically to the researchers. Measures from the second half
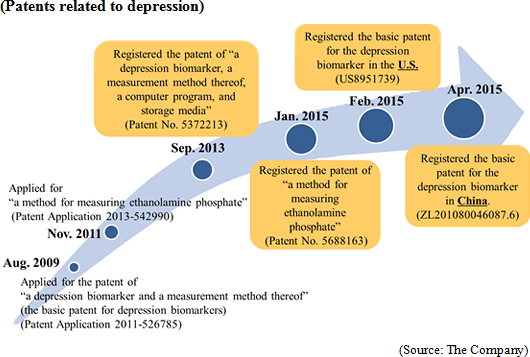
 The company has commenced the development of a blood marker kit for diagnosing depression
On September 28, 2015, the company signed a contract for licensing the exercise of a patent with Sysmex Corporation regarding the development of a blood marker kit for diagnosing depression. The contract period is about 20 years until the expiration of the patent. In the previous term, the company submitted the specifications for the diagnosis kit to Sysmex. Remaining technical problems were solved in the first quarter of this term, and the licensing contract was concluded during the negotiation for preferable licensing. The company will keep proceeding with commercialization. The meanings of this contract, etc. will be detailed in the section "4. Interview with President Kanno." Establishment of revenue base through the promotion of clinical tests for depression
In the previous FY, the Company began to conduct clinical tests for depression in cooperation with specialized hospitals in order to increase recognition on depression biomarkers and expand its business opportunities for fee-based tests for major depressive disorder. The company has signed contracts with 3 hospitals, including Toyoko Keiai Hospital, in Kanto region. Although there are some issues making the business slow down including the burden of the protocol, the company will form more tie-ups with other specialized hospitals, keep increasing specimens, deepen research, in order to have more solid revenue base. |
| Interview with President Kanno |
|
<Biomarker business set in motion>
  <Steadily expanding metabolome analysis business>
<Current state of the company>
|
| Conclusions |
|
However, this decision was not evaluated favorably in the share market. Succeeded to hold a free hand, to be sure, the company needs to show a concrete path definite roadmap for earning profits. On the other hand, it may be necessary to pay a little more attention not only to the potential of the biomarker business, but also to the steady growth of the metabolome analysis business. Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation for investment. The information and opinions contained within this report are made by our company based on data made publicly available, and the information within this report comes from sources that we judge to be reliable. However we cannot wholly guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2016 Investment Bridge Co., Ltd. All Rights Reserved. |





