| A&T Corporation (6722) |
|
||||||||
Company |
A&T Corporation |
||
Code No. |
6722 |
||
Exchange |
JASDAQ |
||
Industry |
Electrical equipment (manufacturing industry) |
||
President |
Shigetaka Misaka |
||
Address |
Yokohama Plaza Bldg. 2-6 Kinko-cho, Kanagawa-ku, Yokohama-shi |
||
Year-end |
End of December |
||
URL |
|||
* The share price is the closing price on August 16, 2017. The number of shares issued was taken from the brief financial report for the latest period. ROE and BPS are those for the previous term.
|
||||||||||||||||||||||||
|
|
* The forecasted values were provided by the company.
This report outlines A&T Corporation, briefly reports the results for the term ending Dec. 2016, and so on. |
| Key Points |
 |
| Company Overview |
|
The company excels at proposing an optimal one-stop solution for preparing necessary products in a laboratory, installing and operating equipment while proposing a layout, and possesses advanced technologies that are highly evaluated by leading overseas OEM clients. In the development process, Tokuyama Corporation formed a business tie-up with Analytical Instruments Inc., which develops, manufactures, and sells clinical test equipment and had been leading the industry by releasing such products as fully automatic blood sugar analyzer in 1978, and in Apr. 1988, they founded a joint venture for distributing their products, A&T Corporation. ("A" of Analytical Instruments and "T" of Tokuyama were combined.) In November 1990, the company established Esashi Factory, which is now the primary production site, in Iwate Prefecture. In 1994, A&T Corporation underwent an absorption-type merger, integrating the diagnosis system division of Tokuyama Corporation. The period from the 1980s to the 1990s was the growth period of the clinical testing industry, in which many core technologies were developed, and the company expanded its business steadily while taking advantage of that trend. In Jul. 2003, the company issued over-the-counter shares. It is now listed in the JASDAQ market of Tokyo Stock Exchange.  ◎ Market scale
According to the Fuji Research Institute Corporation, the scale of the Japanese clinical testing market was 463.9 billion yen in 2015, accounting for 6.0% of the scale of the global market, but the market growth is slight, because the growth of immunoserological tests became sluggish. The market scale is estimated to be 489 billion yen in 2020, with average annual growth rate being only 1.1%. (Domestic and global markets) Based on the information in the website of the Japan Association of Clinical Reagents Industries, A&T Corporation estimated that the scale of the Japanese market of related devices and reagents is about 530 billion yen. The market scales of clinical chemistry and hematology tests are 175.9 billion yen and 27.5 billion yen, respectively. On the other hand, also according to the Fuji Research Institute, the global market scale reached about 62.3 billion US dollars (6.23 trillion yen under the assumption that 1 dollar is equivalent to 100 yen) in 2015, and is projected to keep growing at an annual rate of 2.4%, and reach about 70.4 billion US dollars (7.04 trillion yen under the assumption that 1 dollar is equivalent to 100 yen). It is expected that Eastern Europe, Russia, Asia, South America, and Africa, where the testing environment is still to be developed, will lead the growth. It was mentioned that as the Japanese market was already saturated, Japanese manufacturers would try to operate their business in the global market by utilizing their advanced technology and quality based on their stable business bases in Japan. 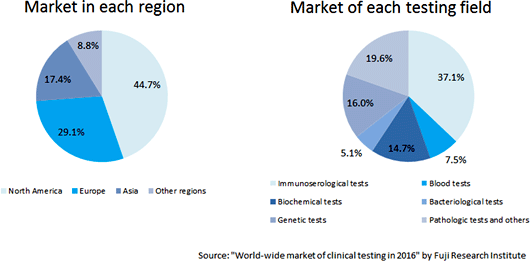 (Trend in each testing field)
Immunoserological testing formed the largest market (37.1%; about 23.1 billion US dollars; about 2.31 trillion yen), and the market of tests mainly for infectious diseases is growing. The blood testing market, in which A&T Corporation operates business, accounts for 7.5% and has a scale of about 4.7 billion US dollars (about 470 billion yen). In North America, Europe (especially western Europe), and Japan, blood testing has been already diffused, and the market growth is stagnant or slightly increasing. In Asia and other regions, the market is growing, and expected to keep growing steadily, according to the survey. (Trend of IVD devices)
According to "Statistical Survey on Trends in Pharmaceutical Production" by the Ministry of Health, Labour and Welfare, the scale of the Japanese medical products market (domestic production amount) in 2015 was about 1.9 trillion yen. Products for medical treatment is dominant, and medical Analyzers, which is handled by A&T Corporation, has a market scale of about 180 billion yen.While there is a significant excess of imports of the overall medical product, there is an excess of exports of IVD devices. This indicates how competitive Japanese companies are. Hitachi and Toshiba supply testing equipment to Roche in Switzerland and Abbott in the U.S., respectively. Likewise, A&T Corporation supplies OEM products to Siemens. Namely, testing equipment made in Japan is now indispensable in the global clinical testing field. 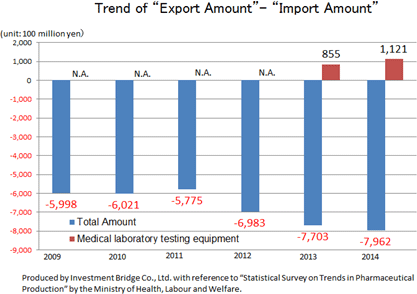 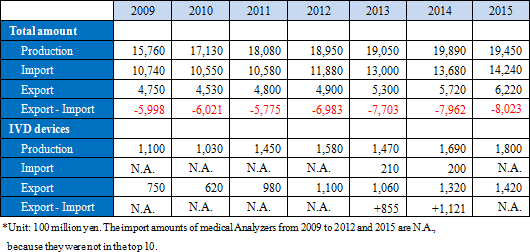 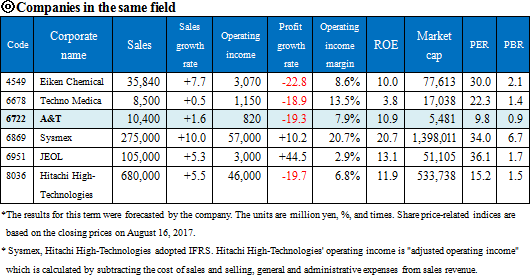 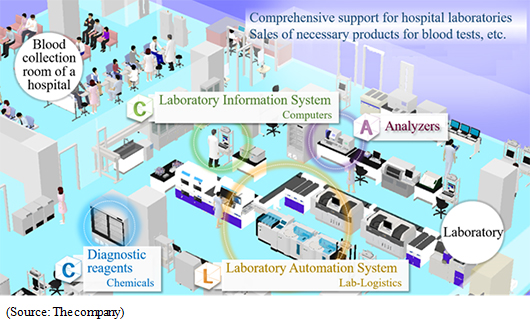 (What is clinical testing?)
Clinical tests can be classified into "biopsies" for directly examining the body with medical equipment, such as X-ray equipment, CT, MRI, electrocardiographic and ultrasonic equipment, and "laboratory tests" for examining biological samples (specimens), such as blood, urine, stool, and cells, collected from patients. A&T Corporation handles products used for laboratory testing, especially blood tests. There are a variety of blood tests conducted at hospitals and in comprehensive medical checkups, including the tests of the hepatic system, the renal system, uric acid, the lipid system, glucose metabolism, blood cells, and infectious diseases. A&T Corporation mainly conducts business related to "electrolyte tests" and "glucose tests." "Electrolyte tests"
The water content constitutes about 60% of the human body, as body fluids, including intracellular fluid and blood plasma. Body fluids are classified into electrolytes, which are mineral ions that dissolve in water and conduct electricity (such as sodium, potassium, calcium, and chlorine), and non-electrolytes, which dissolve in water, but do not conduct electricity (such as glucose and urea). Each electrolyte takes important roles for keeping human beings alive while maintaining a healthy balance -"sodium" adjusts the water content of the body, "potassium" controls muscles and nerves, "calcium" forms bones and teeth, conveys nervous stimuli, and coagulates blood, and "chlorine" supplies oxygen to the inside of the body. If the concentration of electrolytes in blood is abnormal, there is a possibility that the kidneys or hormones are malfunctioning. The purpose of electrolyte tests is to measure the concentration of each electrolyte ion in body fluid, detect the disruption of a balance, and then diagnose a disorder in the body. Sampled blood and urine are examined with testing device.  "Glucose tests"
The sugar in blood plasma (blood sugar) is composed mostly of glucose, which is the only energy source for the central nervous system, including the cerebrum. When the stomach is empty (over 5 hours after eating), the liver emits about 8 grams of glucose per hour, and the brain consumes about half of them, and muscles and red blood cells consume one fourth of them, respectively. Blood sugar level in its normal condition is strictly controlled while keeping a balance between the increase through the absorption from the intestine and the generation in the liver and the decrease through the consumption in the muscles. If this control does not work properly, hyperglycemia or hypoglycemia will occur. A glucose test is conducted for measuring the concentration of glucose in blood or urine.  1. Business Field
The business of A&T Corporation is composed of the "blood testing business," in which the company develops, manufactures, and sells clinical testing devices, reagents, supplies, etc. for blood tests, and the "IT and automation support business," which facilitates the streamlining of manual work in hospital laboratories with IT and automated systems. The company comprehensively supports hospital laboratories. (Since this company conducts this business only, neither its brief financial reports nor securities reports contain segment information. It should be noted that the company discloses the sales of each product series in reference materials for briefing results, etc., but not the sales of each type of business.) 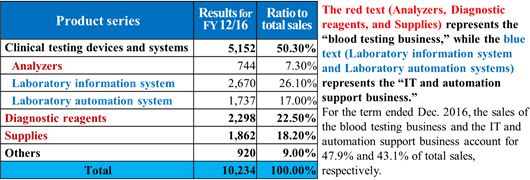 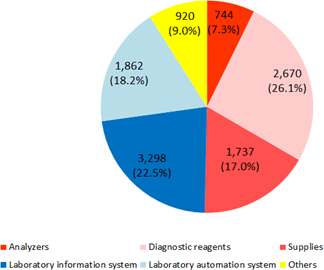 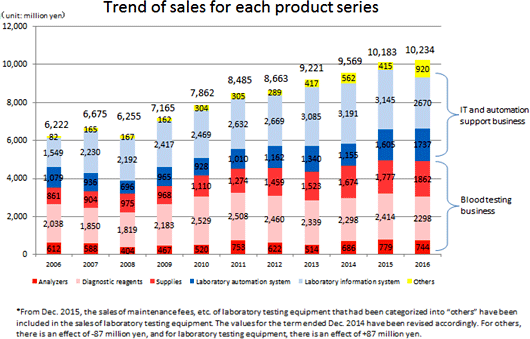 (1) Blood testing business
(Outline)
The company works on developing, manufacturing, and selling Analyzers for "electrolyte tests" and "glucose tests," reagents for clinical tests (for measuring the concentrations of electrolytes, blood sugar, etc.), and supplies (such as sensors installed in analyzer), and offers customer support.
 (Commercial distribution)
*Inside JapanThe company directly sells analyzers, reagents, and supplies to small and medium-sized hospitals via 8 branches nationwide. As of now, about 4,000 units of equipment are in operation. *Outside Japan The company sells analyzers as an OEM. It supplies electrolyte units, which are the specialty products of the company, to other Japanese manufacturers, including JEOL (6951, 1st section of TSE). The OEM clients combine the unit with large-size clinical chemistry analyzers and sell them. As an OEM, JEOL supplies products to Siemens, which is one of global enterprises handling large-size clinical chemistry analyzers. (Business model)
Once Analyzers is newly installed, clinical reagents and supplies will be continuously delivered, and the maintenance service for the equipment will be offered. Once adopted, it is rare for client hospitals to change manufacturers considering the continuity of test data and usability, and so it is difficult for new manufacturers to enter the market. 7 to 10 years later, upgraded models will replace them. This characterizes this business field. (Major enterprises in this field)
Sysmex (6869, 1st section of TSE), Hitachi High-Technologies (8036, 1st section of TSE), JEOL (6951, 1st section of TSE), Wako Pure Chemical Industries (unlisted), ARKRAY (unlisted)
(2) IT and automation support business
(Outline)
In the case of blood tests, it is necessary to convey patient's blood (specimen) sampled in a blood collection room to a clinical laboratory and manually set the specimen at testing equipment. As several kinds of tests need to be conducted for many specimens at the same time, this work is extremely labor-intensive and inefficient, and the human error of taking a wrong specimen is difficult to avoid. In these circumstances, A&T Corporation supports the streamlining of the testing process with the following 2 systems.   Through the introduction of LIS, it became possible to put together the data of test results, which had been printed out for each test item, and give feedback to medical doctors swiftly and accurately. In addition, the data mining function is helpful for reducing the number of times of abnormal value retesting and the duration of testing. (Commercial distribution)
*Inside JapanTargeting the laboratories of medium and large-sized hospitals, the sales division of A&T Corporation sells LIS in cooperation with hospital information system manufacturers, including Hitachi, IBM, and Fujitsu, and LAS in cooperation with large-size clinical chemistry and immunoassay analyzer manufacturers, including Hitachi, Toshiba, and JEOL, as comprehensive solutions *. *For the details of comprehensive solutions, see the section "1-5 Characteristics and Strengths." *Outside Japan The company sells LAS directly to Asia, including South Korea and China. In the U.S., the company sells blood aliquotting instruments to business partners as an OEM. (Business model)
In addition to the maintenance service of LIS and LAS after their installation, the company can connect additional systems, customize the system, and so on for LIS, and can offer maintenance services, sell supplies, and so on for LAS. For both of the systems, stable sales can be expected. Like Analyzers, clients are rarely motivated to shift to other manufacturer's equipment, considering usability, data continuity, etc. The price range per transaction is 10 to 50 million yen for LIS, and 10 to 100 million yen for LAS. (Major enterprises in this field)
LIS: Sysmex CNA (subsidiary of Sysmex), local vendors, etc.LAS: IDS (unlisted), Hitachi-Aloka (unlisted), Siemens, etc. 2. Development systems
A&T Corporation adopted matrix management combining a wide array of products and element technologies for electrical, mechanical, chemical, and information systems that have been nurtured for many years. The company is developing products for offering comprehensive solutions related to C.A.C.L. beyond product categories. About 80 staff members are employed at the headquarters and Shonan Office. R&D cost for the term ended Dec. 2016 was 989 million yen and the ratio of R&D cost to sales was 9.7%. The company will continue active R&D with the ratio of R&D cost being around 10%. 3. Production systems
There are two production sites: Shonan Factory, in Kanagawa Prefecture, for manufacturing clinical reagents and supplies and Esashi Factory, in Iwate Prefecture, for producing equipment and Laboratory Automation System (LAS). The company manufactures high-quality, safe products with advanced equipment under rigorous management. In cooperation with the development section, the company is striving to improve quality and streamline operation. In order to develop the foundation for expanding sales further, the company will construct a new building with a total floor area of 7,300 m2 at Esashi Factory by investing 1.7 billion yen, and strengthen capabilities considerably. 4. Sales routes and methods
As mentioned above, A&T Corporation sells its products to client hospitals via 8 branches in Japan, by utilizing its capability of proposing comprehensive solutions. Outside Japan, the company supplies products to overseas clients and dealers including Siemens through domestic OEM partners such as JEOL. To expand its business scale by supplying products as an OEM like this is the basic strategy, and the company concentrates on the diversification of OEM clients. The direct overseas sales to overseas clients and dealers and its ratio for the term ended Dec. 2016 were about 800 million yen and 7.4%, respectively. But, as the ratio of virtual overseas sales, including the (estimated) overseas sales via domestic OEM clients, for the term ended Dec. 2015 and for the term ended Dec. 2016 was 23.4%, 24.7% respectively, the ratio of overseas sales is in upward momentum 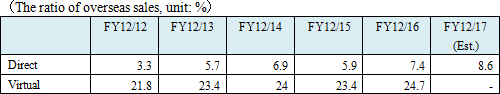 ◎ Capability of proposing comprehensive solutions
A&T Corporation handles products mainly for electrolyte and glucose tests, and does not handle products for other tests. However, client hospitals need to install a variety of testing instruments in their clinical laboratories. To meet their needs, the Laboratory Automation System (LAS) has an automatic conveyor line that is compatible with not only its own products, but also other manufacturers' instruments. There are few manufacturers that possess technologies for producing systems for connecting their own products and other manufacturers' products freely and conveying them. Accordingly, the company occupies about 30% of the Japanese market. The sales staff of the company not only delivers equipment, but also proposes a layout for the most efficient testing with 3D CAD or the like, while considering the area and shape of a laboratory. All above, the company can offer optimal one-stop solutions for preparing necessary products in a laboratory, installing and operating equipment while proposing a layout. This is highly evaluated by client hospitals. ◎ Advanced technologies in specific fields
A&T Corporation handles products mainly for "electrolyte tests" and "glucose tests." Especially, its advanced technology for electrolyte analyzers can be verified by the fact that its products are supplied to JEOL, which is a leading manufacturer of measurement devices, including medical instruments, and Siemens, which is a large global company. As mentioned in the section of the market environment, Japanese medical Analyzers is highly competent in the world, and A&T Corporation contributes to the competitiveness of Japanese products. 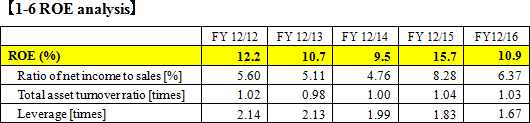 The estimated ratio of net income to sales for this term is 5.4%. |
| First Half of Fiscal Year December 2017 Earnings Results |
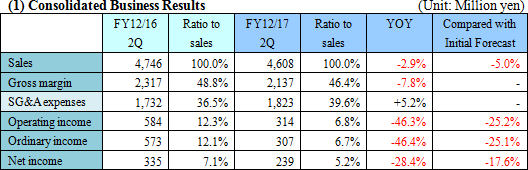 Sales and profit dropped year on year, failing to reach the estimate.
Sales were 4,608 million yen, down 2.9% year on year. The number of large-scale transactions for Laboratory Automation Systems inside and outside Japan increased, but the OEM sales of Analyzers were weak, and the OEM and direct sales of Diagnostic reagents were sluggish. Operating income was 314 million yen, down 46.3% year on year. As the overseas transactions for Laboratory Automation Systems increased, cost of sales rose. In addition, the augmentation of SG&A expenses due to the growth of R&D cost (investment in the development of subsystems for Laboratory Information Systems) was not offset. Neither sales nor profit reached the estimate.
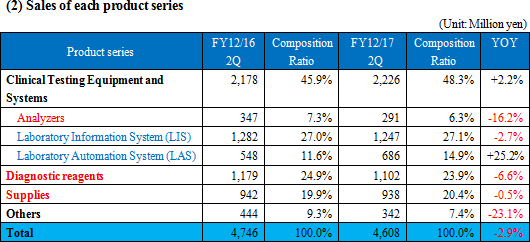 ◎ Clinical Testing Equipment and Systems
Sales grew 2.2% year on year. The sales of Analyzers dropped because one of main OEM partners for the electrolyte unit has changed strategy for its key driver. and prices were revised. The OEM sales outside Japan were healthy. The company considers that it is important to keep seeking new OEM partners for electrolyte, and is approaching two Japanese companies and some Chinese ones. The number of large-scale domestic transactions for Laboratory Information System decreased. As for Laboratory Automation System, the number of large-scale transactions grew inside and outside Japan. Especially, the OEM sales to business partners in the U.S. were favorable. ◎ Diagnostic reagents
Sales decreased 6.6% year on year. Overseas sales were healthy, but the OEM sales were sluggish due to price revision, etc., and domestic direct sales dropped. ◎ Supplies
Sales declined 0.5% year on year. Quantity sold increased slightly, but sales decreased slightly due to the revision to prices for OEM partners. ◎ Other
Sales dropped 23.1% year on year.The sales of purchased products accompanying domestic large-scale transactions for Laboratory Information System and Laboratory Automation Systems decreased. (3) Major strategies and their progress
The enlargement of Esashi Factory is progressing healthily. The completion ceremony is to be held in September 2017, and the production lines for some Diagnostic reagents will be transferred from Shonan Factory to Esashi Factory. In October, the factory will start manufacturing devices. ◎ Equipment investment In April 2018, the company will start manufacturing Diagnostic reagents after obtaining the approval for applications pursuant to the Law on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical devices. In parallel, the company is planning to improve the Shonan production site by utilizing vacant space of Shonan Factory. Through a series of strategies, the production capacities of devices and Diagnostic reagents/supplies are estimated to expand about 1.7 times and 3.4 times, respectively. ◎ Product development
The company is investing in the development of subsystems for Laboratory Information System, with the aim of completing the subsystems by the end of this year and posting their sales from the next term. In the second half of this term, the company will start the full-scale development of large-scale modules (refrigerators) accompanying Laboratory Automation Systems. A prototype will be completed at the beginning of the next year, and the product will be released in the second half of the next term. ◎ Cultivation of new business partners
As mentioned above, the company will seek new OEM partners for electrolyte inside and outside Japan. In addition, the company is negotiating with a Chinese company for the conclusion of a contract for distributorship, with the aim of enriching sales channels for Laboratory Automation System mainly in Asia. ◎ Structure reform of the manufacturing system
The company aims to reduce cost and improve yield through the horizontal expansion of manufacturing cost analysis. In addition, the company launched a project for work-style reforms to improve productivity. 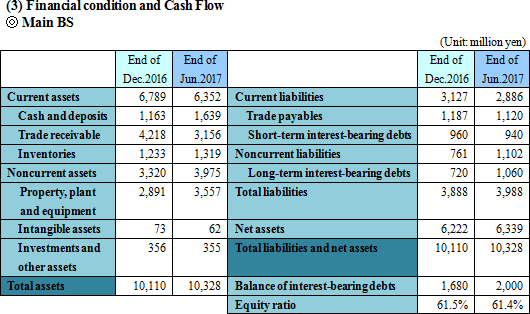 Total liabilities rose 100 million yen from the end of the previous term to 3,988 million yen due to the augmentation of long-term interest-bearing debts. Net assets grew 117 million yen from the end of the previous term to 6,339 million yen due to the increase in retained earnings. As a result, equity ratio was 61.4%, nearly unchanged from 61.5% at the end of the previous term. 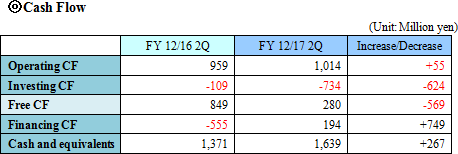 |
| Fiscal Year December 2017 Earnings Estimates |
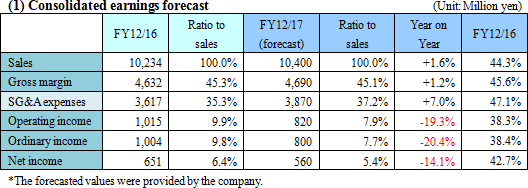 No revision to the earnings forecast. Sales are estimated to increase, while profit is forecasted to decline, due to upfront investment.
There is no revision to the full-year earnings forecast. Sales are estimated to be 10.4 billion yen, up 1.6% year on year. The OEM sales of the blood testing business and the Laboratory Information System, CLINILAN GL-3, which were both sluggish in the first half, are expected to recover in the second half. Gross profit rate is forecasted to decline 0.2 points, because of the augmentation of cost due to the equipment investment for the factory relocation. Operating income is projected to be 820 million yen, down 19.3% year on year, because SG&A expenses are estimated to increase due to the upfront investment in the development of subsystems for Laboratory Information Systems and large-scale modules for Laboratory Automation Systems. The development cost of Laboratory Information Systems is for intensive investment, which is to be posted in this term only. The dividend amount is to be 20 yen/share, unchanged from the previous term. The estimated payout ratio is 22.3%. 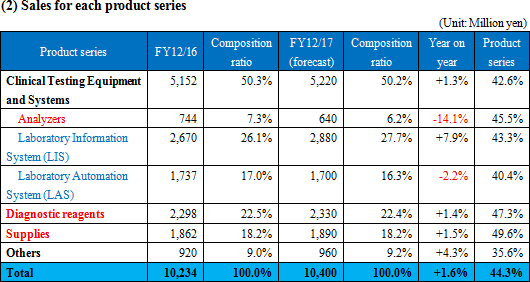 The following changes from the previous term are forecasted. |
| Business operation from now on |
     <Strategies in China>
(Market environment)The scale of the clinical examination market in China exceeds 30 billion yuan (= 500 billion yen), and its annual growth rate is 15-20%. The company estimates that the scale of the biochemical testing market, which is the major battlefield for the company, is about 10 billion yuan (= 167 billion yen). The current market scale is nearly equal to that in Japan, but is expected to grow considerably. It is considered that there are about 14,300 hospitals in China, and the company targets medium to large-sized hospitals that have over 100 beds, and the number of such hospitals is about 8,300. The needs for advanced medical care are growing, and the number of hospitals is increasing year by year. On the other hand, in the clinical examination market in China, European and U.S. companies have occupied overwhelming shares so far, but Chinese manufacturers are growing significantly, improving their technological levels and changing business environments considerably. (Business strategies) In 2012, the company established a joint venture, to sell Analyzers (electrolyte units), and has conducted the OEM sales to one Chinese manufacturer, but considering that it is necessary to operate business in a more multifaceted manner, in order to cultivate the huge market amid the change in the market environment, the company obtained a new business license for the joint venture in June 2016, and established a division for promoting the business in China, to develop a system for carrying out full-scale business in China, and opened an expatriates' office in Shanghai in September 2016. The expatriates' office in Shanghai strives to glean information more meticulously. Conducting business only by themselves in the Chinese testing market is accompanied by significant risk, because the preference to products manufactured in China is growing and there is a difference in distribution channels. Accordingly, it is difficult to do business without a partner, such as a joint venture, and so the company is looking for an adequate partner, and about to find some promising candidates. *Analyzers, electrolyte units Currently, the company conducts the OEM sales to a leading Chinese manufacturer, and is approaching some manufacturers of biochemical analyzers as new OEM partners for alliances. * Laboratory Automation System In the previous term, the core product, CLINILOG V4, was installed in a large hospital in China for the first time. The laboratory of this hospital serves as a showroom, where many hospital personnel and enterprises can see the actual operation of CLINILOG V4. The company is now negotiating with a candidate distributor for the conclusion of a contract. The company possesses the capability of comprehensively offering optimal solutions ranging from installation to operation, providing laboratories with necessary products and designing layouts. By utilizing this strength also in the Chinese market, the company plans to cultivate the huge market. |
| Conclusions |
|
Fortunately, there is no revision to the earnings forecast, and the company expects the recovery in the second half. The progress of the cultivation of new OEM partners is noteworthy. Meanwhile, we would like to pay attention to the release of the second Laboratory Automation System in the Chinese market.  |
| <Reference: Regarding corporate governance> |
 ◎ Corporate Governance Report
Last Update: March 29, 2017.As a JASDAQ listed company, the company fully follows the 5 items of basic principles of the corporate governance code. Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation for investment. The information and opinions contained within this report are made by our company based on data made publicly available, and the information within this report comes from sources that we judge to be reliable. However we cannot wholly guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright (C) 2017 Investment Bridge Co., Ltd. All Rights Reserved. |




