| CellSeed (7776) |
|
||||||||
Company |
CellSeed Inc. |
||
Code No. |
7776 |
||
Exchange |
JASDAQ |
||
Industry |
Precision Instrument (Manufacturing) |
||
President |
Yukio Hasegawa |
||
HQ Address |
Haramachi 3-61, Shinjuku-ku, Tokyo |
||
Year-end |
December |
||
URL |
|||
* Stock price as of closing on 2014/2/28. Number of shares at the end of the most recent quarter excluding treasury shares.
|
||||||||||||||||||||||||
|
|
We present this Bridge Report along with an analysis of the fiscal year December 2013 earnings results for CellSeed Inc.
|
|
| Key Points |
 |
| Company Overview |
|
<Business Description>
CellSeed's business can be divided into two main divisions including "cell sheet regenerative medicine business," where various types of cell sheet tissues are developed, fabricated and sold, and "regenerative medicine support business," where temperature -responsive cell cultureware used in the fabrication of "cell sheets" are produced and sold.In the "cell sheet regenerative medicine business division", research and development for several new regenerative medicine products is being conducted jointly with research partners.
<Superiority of CellSeed's Technological Foundation>
Currently, the key issue for "cell sheet regenerative medical technologies" of artificially fabricating coherent tissue from human cells has been fundamentally resolved. Furthermore, proof of concept for corneal regeneration epithelial cell sheet, cardiac muscle regenerative patch, periodontal tissue regenerative sheet, and regenerated cartilage sheet in the cell sheet regenerative medicine business division has been shown in human clinical trials for a wide variety of tissues and conditions. In addition, the various applications of the above mentioned cell sheet regenerative medicine products are unprecedented, because most of the regenerative medical products launched in the market thus far are either skin or cartilage.
<Business Environment>
The industrialization of regenerative medicine is benefitting from favorable environmental conditions, as the Prime Minister Abe has taken steps to facilitate the legal structure invigorate Japan's regenerative medicine industry. For example, the Regenerative Medicine Promotion Law was established in April 2013 to realize the commercialization of regenerative medicine, and in November 2013 the Revised Pharmaceutical Law (Medical Products and Equipment Law) and the Regenerative Medicine Safety Law were established. The Pharmaceutical and Medical Equipment Law newly defined "regenerative medicine and medical products" in addition to medical products and equipment, and introduced systems that take these definitions into consideration (Example: Special approval system with special conditions and time limits, known as an early approval system). Furthermore, the Regenerative Medicine Safety Law is designed to secure safety in the three categories of regenerative medicine, by introducing safety regulations according to risks and newly establish cell processing technologies (The outsourcing of the fabrication process for specially processed cells).
|
| Progress in FY12/13 Business Strategies (Midterm Business Plan: FY12/13 to FY12/15) |
|
(1) Creation and Promotion of Midterm Business Plan (FY12/13 to FY12/15)
The midterm business plan announced in February 2013 is comprised of three cornerstones and designed to promote new growth business models. Moreover, the midterm business plan is based upon a rolling system that calls for review of the plan every term.
Progress in Implementing Business Strategies
・Modification of corneal regeneration epithelial cell sheet development strategy
Along with the fast paced facilitation of the environment for the industrialization of regenerative medicine, CellSeed has chosen to shift the focus of its development activities to Japan. Specifically, CellSeed expects to leverage business alliances and public subsidies and assistance for its development activities within Japan. At the same time, in March the Company has temporarily withdrawn its sales approval application submitted to the European Medicines Agency (EMA) in Europe, in light of the results of its deliberations with the EMA and the facilitation of the environment to bring about industrialization of regenerative medicine within Japan. However, the possibility of securing approval in the future remains in place, and currently CellSeed is considering business alliances to bring about commercialization. At the same time, efforts to promote joint development and commercialization with Emmaus Medical are being promoted in the United States (Two contracts have been consolidated to become one in order to be able to focus upon the cornea realm).
・Endeavors for Corneal Epithelial Regenerative Cell Sheet within Japan (A Project Consigned from METI)
Within Japan, two projects including 1) "global standard for corneal regeneration epithelial cell sheet medical products effectiveness evaluation method" and 2) "cell sheet regenerative medical product manufacturing cost and quality evaluation methods" have been consigned by the Ministry of Economy, Trade and Industry's (METI) to CellSeed in fiscal year 2013 as part of the "Regenerative Medicine Industry Promotion Projects." The first project has been consigned to CellSeed based upon its track record in the area of regenerative medical product screening in overseas markets, and the second project has been consigned for the Company's experiences in the regenerative medicine support business. In the pipeline for autologous mucosal cell epithelial cell sheets, the experience and knowhow gained with corneal regeneration epithelial cell sheets can be leveraged. In addition, the results of the first project can be used in common applications of cell raw material for autologous mucosal cell epithelial cell sheets, while the results of the second project may be applied to automated fabrication (allowing for fabrication cost reductions and securing quality) in various pipeline products. The implementation period of the contract is from its signing until March 31, 2014.
Reference: Develop an "Efficacy Evaluation Method for Corneal Regeneration Epithelial Cell Sheet as a Global Standard"
The efficacy evaluation method for corneal regeneration epithelial cell sheet is currently limited to qualitative evaluation, and the establishment of a rational quantitative evaluation method is critical. Therefore, the ability to objectively evaluate the stability of reduction in conjunctiva in the axis of sight due to recovery of the epithelial layer and the overcoming of inhibition of cornea angiogenesis will be validated in this project .Fluorescein stain test is implemented at 12 months after surgery to validate the stability of reduction in conjunctiva in the axis of sight due to recovery of the epithelial layer, and quantitative analysis using image analysis of the existence of conjunctiva on the surface of the cornea is employed (Baseline: Immediately after transplant). Also, the standard of efficacy can be determined by quantifying the level of conjunctiva in the axis of sight, by the restraint of conjunctiva through recovery of the corneal epithelial layer. 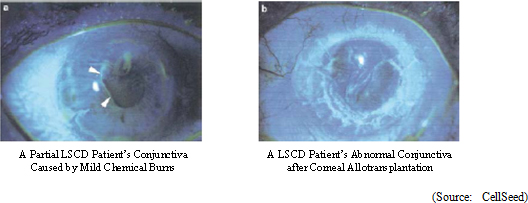 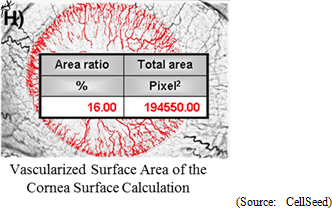
 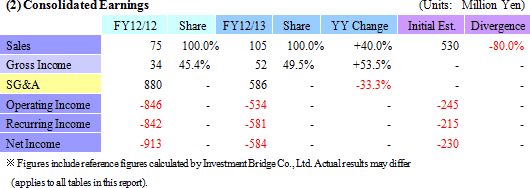 ¥105 Million in Sales, ¥581 Million in Recurring Loss
Sales rose by 40.0% year-over-year to ¥105 million. By business segment, the regenerative medicine support business recorded ¥88 million in sales, a ¥13 million rise from the previous term, and ¥16 million (No sales recorded during the previous term) were booked in the cell sheet regenerative medicine business. With regards to the cell sheet regenerative medicine business, the dissolution of the contract with GENESIS Pharma SA accompanying the review of development plans for corneal regeneration epithelial cell sheet in Europe contributed to the booking of lump sum payments received at the time of the formation of the contract of ¥16 million.An operating loss of ¥534 million was recorded (A loss of ¥846 million was recorded in the previous term). Rationalization efforts to reduce costs and restrain research and development expenses contributed to a ¥293 million or 33.3% reduction in selling, general and administrative expenses to ¥586 million from the previous term. Factors behind Divergence from Initial Estimates
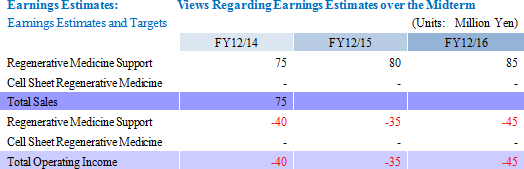
With regards to sales, the inability to acquire ¥350 million in business alliance lump sum payments (alliance negotiations still being conducted), and the inability to book ¥110 million of the lump sum fee received from Emmaus due to the review of the contract, were the main reasons for the divergence in sales from initial estimates.The lower sales contributed to a ¥425 million expansion in operating loss. However, better than expected results in restraining selling, general and administrative expenses contributed to a ¥149 million improvement in operating loss. 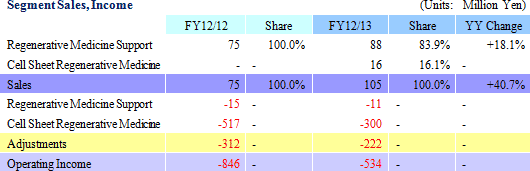 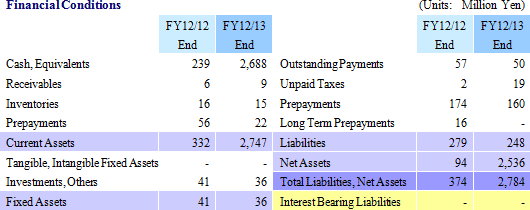 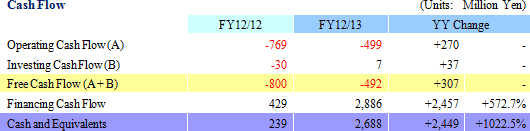 |
| Strategy in FY12/14 (Midterm Business Plan: FY12/14 ~ FY12/16) |
|
(1) Awareness of the Operating Environment
2013 Became an Epoch Making Year as Japan Led the World in Efforts to Industrialize Regenerative Medicine
CellSeed believes that the regenerative medicine industry within Japan is entering a period of full scale development and rapid growth. As a national policy, Japan is promoting the industrialization of regenerative medicine, and the laws outlined below were established and revised during 2013.
Revised Pharmaceutical Law (Established in November 2013, Early Approval System) Regenerative Medicine Safety Law (Concerning the processing industry, established in November 2013) 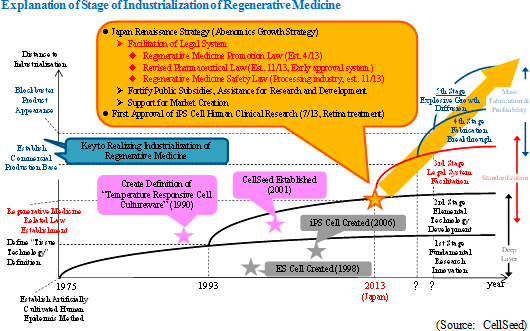 3 "A's" to Realize "Industrialization of Regenerative Medicine": Automation, Allogeneic, Alliances
The key drivers for the industrialization of regenerative medicine include "establishment of foundations for commercial fabrication" and "appearance of blockbuster products." In order to "establish foundations for commercial fabrication," fundamental reductions in fabrication costs, dramatic fortification of fabrication output, and improvements and consistency in product quality levels are critical. In addition, strategic targeting, differentiation and resolution of technological issues leveraging open innovation, and anticipatory investments in marketing to match the characteristics of products for paving the way for "blockbuster products to appear" are also necessary. Furthermore, three key concepts critical to the industrialization of regenerative medicine include "automation," "allogeneic," and "alliance."
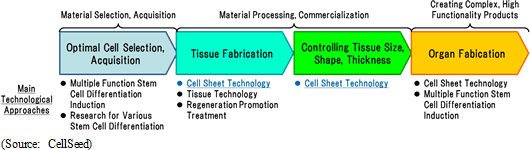
Cell Sheet: A Revolutionary Technology Fundamentally Resolving Various Issues by "Artificially Fabricating Tissue Using Only Human Cells"
Regenerative medicine entails the following processes: optimized cell selection, acquisition → tissue fabrication → tissue size, shape, thickness control → organ fabrication. Furthermore, cell sheet technology entails the technological processes of tissue fabrication, tissue size, shape, thickness control, and organ fabrication. Therefore, in the tissue fabrication process, cell sheet technology fundamentally resolves the issue of "using only human cells to artificially fabricate various tissues". In addition, cell sheet technology has shown successful results in non-clinical research by providing a fundamental solution to the issue of controlling tissue size, shape and thickness (stratification + vascular injection). At the same time, another issue is the fact that organ fabrication can only be done after tissue fabrication is successfully completed.
 (2) Midterm Business Plan Overview (FY12/14 to FY12/16)
Full Scale Industrialization of Regenerative Medicine Road Map: Using "Regenerative Medicine Industrial Package" Developed in Japan to Address the Global Market
In order to promote the diffusion of "cell sheet regenerative medicine" products, developed by using revolutionary regenerative medical technologies created in Japan, for sale and use throughout the world, the early market launch of the first cell sheet regenerative medical product is critical. CellSeed will leverage academic-industrial and business alliances to realize autologous regenerative medicine. Academic-industrial and business alliances will enable CellSeed to reduce the burden of anticipatory investments necessary for commercialization, and supplement the business resources of the Company by providing access to highly skilled staff and cutting edge technologies in realms where it does not have a position of superiority relative to its competitors. Furthermore, CellSeed will endeavor to realize cross regenerative medicine applications as part of the process of full scale industrialization of regenerative medicine (cross materials + fabrication automation).
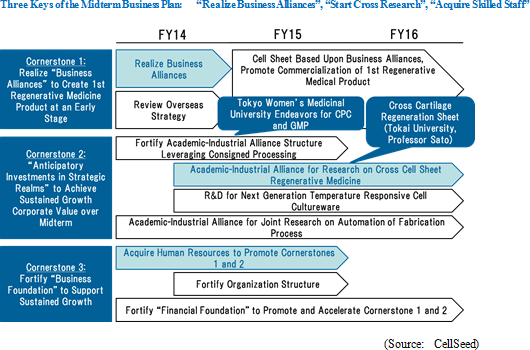
Midterm Business Plan (FY12/14 to FY12/16)
Overview: Fortifying the "Three Cornerstones" of the Midterm Business Plan Announced in February 2013
The following three cornerstones of the midterm vision of "creating new sustainable growth models by leveraging changes in the external environment" will be pursued.
  ・Unforeseen factors that may influence research and development expenses (Changes in research and development activity plans may result from the business alliances) (3) Capital Sourcing: 3rd Party Placement of 1st Uncollateralized Convertible Bond with Stock Options, 12th Stock Option Issuance
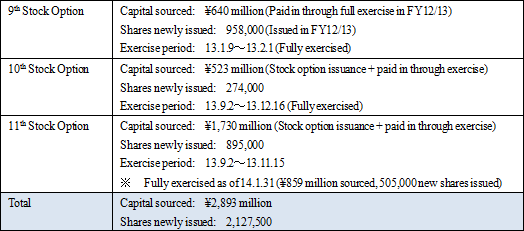
A third party placement of the first uncollateralized convertible bond with stock options (hereafter called convertible bond) and the issuance of 12th stock option (hereafter called stock option) will be conducted. CellSeed expects to use the capital procured from these activities to accelerate its midterm research and development activities and thereby raise its corporate value.Including the capital raised through the exercise of new stock options amounting to ¥2.277 billion, the total capital raised amounts to ¥2.786 billion. The 1st uncollateralized convertible bond with stock options raised ¥500 million, the issuance of the 12th stock option raised ¥9 million, and the future exercise of options is expected to amount to a maximum of ¥2.277 million. Specifically, capital procured will be used for "strategic investments" primarily for cross cartilage cell sheet research and development, personnel expenses to fortify its research and development structure, commercialization of the first cell sheet regenerative medical product, as well as operating capital. 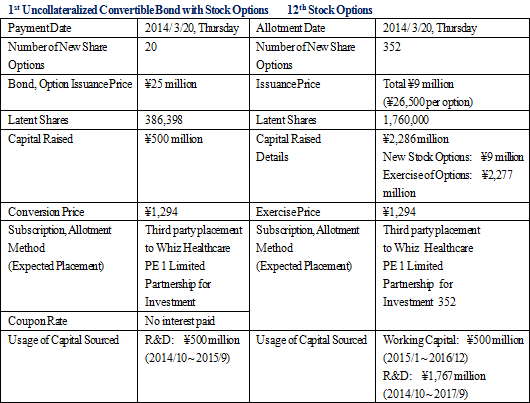 Reason for Selection of Whiz Healthcare for Third Party Placement
Whiz Healthcare PE 1 Limited Partnership for Investment was chosen for the placement in this recent round of funding because Whiz Partners Inc. is one of the executing members of this partnership and the creator of this fund. In addition, Whiz Partners has made several investments in Japan's bio-ventures, with its first full scale investment in the bio and healthcare realms being made in 1999, and so far it has made investments in about 30 bio and healthcare related firms throughout the world (Japan, United States, Germany, France, Israel, Korea and other countries). Furthermore, CellSeed has confirmed the wealth of knowledge and experience of Whiz partners management in not only the bio and healthcare industries but also in financial instruments transactions (Registered with Kanto Local Finance Bureau, License Number 2590) through interviews with the company. Whiz Partners also boasts a strong financial position with no interest bearing liabilities and a high level of credibility as an independent fund operator. In addition, the declared goal of Whiz Healthcare PE 1 Limited Partnership for Investment to "contribute to the preservation of life" is in keeping with the business policy of CellSeed's various businesses. For these reasons, CellSeed has chosen Whiz partners as the third party partner with which to place its bond and stock options.
Holding Policy for the Third Party Placement
Whiz Healthcare PE 1 Limited Partnership for Investment by policy does not expect to maintain the shares of CellSeed over the mid to long term, and is expected to sell the shares in response to market trends, demand from investors, and based upon the policies of business partners. At the same time, the intention of Whiz Partners is not merely to recoup its investment, but to sell shares to investors with which synergies can be derived and/or who can become stable shareholders with the goal of optimizing the capital structure of CellSeed and to raise its valuation in the equity market.Whiz Healthcare PE 1 Limited Partnership for Investment as the operator responsible to members participating in the fund will pay close attention to the impact of its activities in the market, and abide by regulations regarding insider trading in any activities when selling acquired shares . |
| Conclusions |
|
Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2014, All Rights Reserved by Investment Bridge Co., Ltd. |




