| CellSeed (7776) |
|
||||||||
Company |
CellSeed Inc. |
||
Code No. |
7776 |
||
Exchange |
JASDAQ |
||
Industry |
Precision Instrument (Manufacturing) |
||
President |
Setsuko Hashimoto, Ph.D. |
||
HQ Address |
3-61 Haramachi, Shinjuku-ku, Tokyo |
||
Year-end |
December |
||
URL |
|||
* Stock price as of closing on 2015/3/12. Number of shares at the end of the most recent quarter excluding treasury shares.
|
||||||||||||||||||||||||
|
|
We present this Bridge Report along with analysis of the fiscal year December 2014 earnings results for CellSeed Inc.
|
|
| Key Points |
 |
| Company Overview |
|
<Cell-sheet Regenerative Medical Technologies and Cell-sheet Regenerative Medicine>
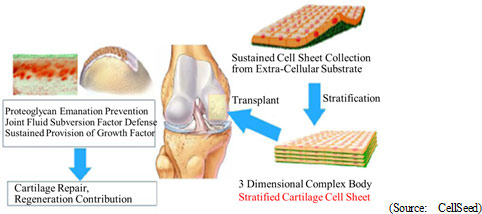
"Cell-sheet regenerative medical technologies" are the general-purpose platform technology for regenerative medicine. Cells are taken from the patient to be cultivated into sheet form for use in various treatments. However, the most revolutionary aspect of "cell-sheet regenerative medical technologies" is the use of temperature-responsive cell cultureware equipment that enables the collection of undamaged cells, which had previously been impossible. Cells are normally cultivated (proliferated) in culture dishes and multiply while adhering to the surface of culture dishes. When cells are collected, they are stripped from the dish using trypsin and other hydrolase proteins, a method that may result in damage of the protein surface of the cells (extracellular matrix) and deformation on living cells (cell damage). Therefore, these damaged cells cannot graft or function properly.On the other hand, temperature-responsive cell cultureware can be used to naturally collect cells from the surface of the culture dish by altering the temperature to change cell adhesion characteristics, allowing for the collection of cell-sheets that are close to their natural condition with cell protein characteristics remaining undamaged (Possible to create tissue, organs close to their natural conditions). In addition to filling the space between cells, cell surface proteins (extracellular matrices) also play a structural role and provide a foothold for cells to bond and allow cells to propagate while controlling division. This is a critical substance to allow cells to function as cells, and contribute to the repair (Regeneration) of the affected area. 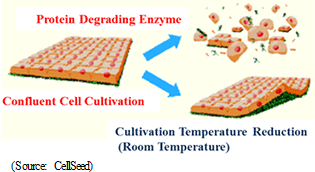 By reducing the cultivation temperature, the characteristics of temperature-sensitive polymers are changed to allow cell-sheet detachment without damaging the cell surface protein (Extracellular matrix). Traditionally trypsin and other protein type hydrolytic enzyme procedures were used. However, the protein type hydrolase degradative enzyme procedures were factors in the subversion of fusing cells and adhesion, leading to significant damage of cells.
|
| Market Environment and CellSeed's Mission |
|
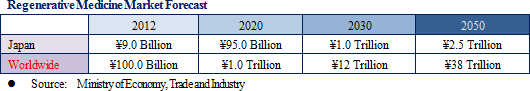
(1) Growth Market: Regenerative Medicine Market Expected to Reach ¥2.5, ¥38 Trillion in Japan, Overseas respectively in 2050
 (2) Facilitation of an Environment for Diffusion of Regenerative Medicine
Regenerative Medicine Founding Year
The pharmaceutical industry has evolved from pharmaceutical compounds created from chemical synthesis (Aspirin is the first medicine) at its start around 1900, to antibody based pharmaceuticals made from genetically modified proteins around 1980, which led to the birth of "biotechnology-based pharmaceuticals". Thereafter, revolutionary technologies including drugs called "gene medicines" made from genes, cancer immunotherapy that use "cell-based pharmaceuticals" made from living cells, and "tissue-based pharmaceuticals" treatments that use living human tissue (Regenerative medicine) have been created (The regenerative medicine products specified in the Pharmaceuticals and Medical Devices Act and the Act of Ensuring Safety of Regenerative Medicine, etc. include not only "gene medicines," "cell-based pharmaceuticals," but also "tissue-based pharmaceuticals.").In addition to the challenging technology and cost issues, no supportive establishment of legal systems or policy reviews were available (Legal restrictions based upon the assumptions of pharmaceutical compounds acted as barriers to the diffusion of biotechnology based pharmaceuticals) at the time when "biotechnology-based pharmaceuticals" first appeared. At that time establishing the market was questioned, however, the current sales of biotechnology-based pharmaceuticals accounts for 30% of the total pharmaceutical product sales after 30 years. The technological and cost hurdles of "tissue-based pharmaceuticals" remain in place, but Prime Minister Abe's administration has identified these developments as a strategic growth realm, and has therefore made legal revisions designed to promote the diffusion of and facilitate the environment for "biotechnology-based pharmaceuticals", including the introduction of an "early approval system" in the Pharmaceuticals and Medical Devices Act and the permission of "outsourcing cell cultivation" in the Act of Ensuring Safety in Regenerative Medicines, etc.. These approaches seem to make faster progress of diffusing "tissue-based pharmaceuticals" than "biotechnology-based pharmaceuticals." 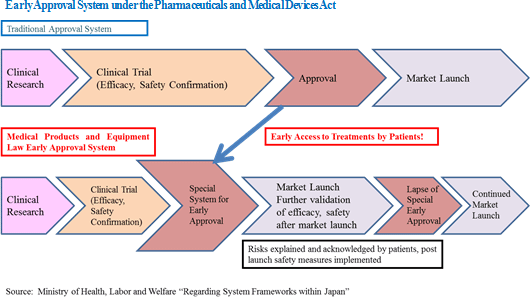 CellSeed's Mission
The Japan-originated "cell-sheet engineering," which is the basic technology of CellSeed's business, has come to the forefront on global basis as the world's first platform technology that will continuously create tissue-based medicine among the regenerative medicines. CellSeed's strength is its business that covers all the value chain of regenerative medicine, including intelligent cell culturewares, cell-sheet cultivation, and treatment with regenerative medicine. With these background, CellSeed set three objectives as its Missions; (1) bringing regenerative medicine to patients (2) introducing the unique platform technology of "cell-sheet engineering" globally (3) initiating health innovation by providing safe and high quality products. CellSeed aims to accelerate its growth by implementing these missions and predicting the movements of the future medical industrialization of regenerative medicine, and to shift from a company supporting clinical development of the basic research at universities to a new stage as a company to realize regenerative medicine and to generate profits. 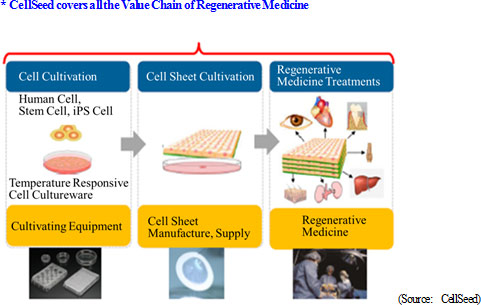 Intelligent Cell Cutureware Business
As described earlier, it is possible to collect cultured cells in a living state using CellSeed's revolutionary cell culturewares. "Temperature-responsive" types are exclusively produced and sold by CellSeed on global basis. Previously, these intelligent cell cultureware have been strategically sold to universities and research institutes as tools to develop partners rather than seeking profit from this segment. However, the new management team formed in June 2014 found its high potentials and placed this segment as important and potential commercial products for earning income. CellSeed plans to expand the product line up to meet various requirements in the market. CellSeed has made partnership agreement with Dai Nippon Printing Co., Ltd. for volume production while CellSeed will dedicate the development and sales of these product area.Cell-sheet Manufacturing Business The legislative changes particularly in the Act of Ensuring Safety in Regenerative Medicine, etc., enabled outsourcing to produce and provide cell-sheets. CellSeed still prioritizes the development of its cultureware products, but has an idea to expand the business for culturing cell-sheets for the future outsourcing needs based on the extensive experiences. Regenerative Medicine Treatment Business
CellSeed has identified epithelial cell-sheet for esophageal regeneration and regenerated cartilage sheet as prioritized pipelines, aiming for fast commercialization of these as its first product in the market. CellSeed also aims for starting clinical trial in Japan and Sweden for the epithelial cell-sheet for esophageal regeneration in the latter half of fiscal 2015, and for autologous regenerated cartilage sheet in the fiscal 2016.
|
| Review of Fiscal Year December 2014 |
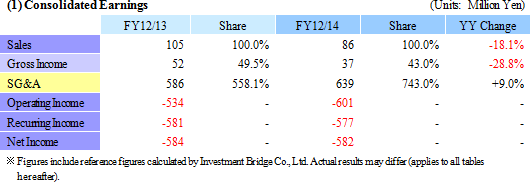 With regards to the regenerative medicine support business, the outsourcing of the fabrication function of temperature-responsive cell cultureware to the collaborative partner Dai Nippon Printing Co., Ltd. (DNP) was undertaken to allow CellSeed to focus its resources upon the development and sales functions (The Tomioka Plant was closed along with the completion of transferring its technology and equipment in December 2014.). This arrangement should enable the Company to respond quickly and flexibly to increases in demand for equipment. In addition, CellSeed will promote development and expand sales of products which are adapted to the outlook for increases in clinical development applications. With regards to the cell-sheet regenerative medicine business, consultations with the Pharmaceuticals and Medical Devices Agency (PMDA), which is responsible for providing guidance and advice to manufacturers for the purpose of improving safety of pharmaceuticals and medical equipment, have been conducted as a part of preparations for the commercialization of cell-sheet regenerative medicine products at an early stage. 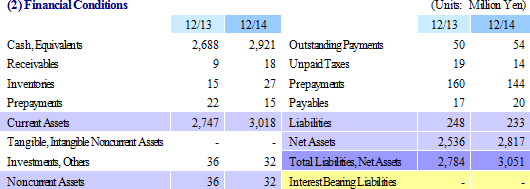 |
| Mid-termMid-termMid-term Business Plan (FY12/15-17): Develop Cell-sheet Regenerative Medicine, Achieve Global Diffusion |
|
<Mid-termMid-termMid-term Business Plan Overview>
In light of the enforcement of the new law regarding regenerative medicine, CellSeed has identified Japan as the top priority hub of cell-sheet regenerative medicine development. Consequently, efforts to achieve commercialization of regenerative medicine products at an early stage and to fortify development and sales of temperature-responsive cell cultureware will be implemented as a means of securing earnings. In-house development of cell-sheet regenerative medicine product pipelines have been identified, including as high priority, with development of cell-sheet regenerative medicine pipelines to be expanded on a global basis.
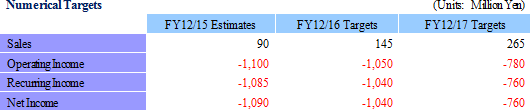 With regards to profits, increases in clinical trial related expenses, labor expenses arising from fortified marketing functions, and developmental expenses due to new product development for temperature-responsive cell cultureware are anticipated. In addition, the CPC is expected to begin operations from fiscal year December 2016 to 2017, and subsequent depreciation expense from the start of operation has been incurred into operating expenses from fiscal year December 2017. At the same time, scale merit is expected to be derived from an increase in sales and the transfer of fabrication of temperature-responsive cell cultureware to DNP are expected to lead to a significant improvement in profitability, supposedly exceeding the increase in sales.
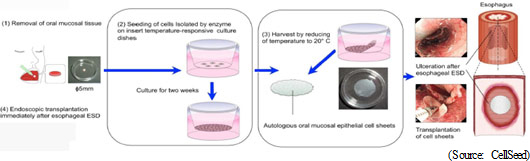 A fundamental development agreement for cell-sheet regenerative medicine has been formed with "Cell-sheet Tissue Engineering Center (CSTEC) of Tokyo Women's Medical University's Institute of Advanced Biomedical Engineering and Science," which is the project of "Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program" for Developing Innovation Systems as part of the Ministry of Education, Culture, Sports, Science and Technology Promotion Adjustment Expenses," headed by Professor Teruo Okano, the principal proponent of esophageal regeneration epithelial cell-sheet clinical application development. For the purpose of promoting the development of esophageal regeneration epithelial cell-sheet business in Europe, CellSeed Sweden AB was established in Sweden in May 2015. In parallel with clinical trials, the Company will promote the development of cell-sheet transplant devices (since transplant surgery of cell-sheet for esophageal regeneration requires highly sophisticated technique, the development of specialized devices is anxiously awaited to reduce the burden on doctors) and market the devices as a combination product of an epithelial cell-sheet for esophageal generation. DNP which performs contracted manufacturing of temperature-responsive cell cultivation equipment products, is a participating company of CSTEC. Market Size
According to the estimate by CellSeed that is based on the information on the cancer patients published by Center for Cancer Control and Information Services, National Cancer Center, as well as the survey conducted by Nikkei Newspaper on "the 47 Hospitals that have performed over 80 cases surgery for esophageal cancer patients", there were 20,700 esophageal cancer patients in 2012, and approximately 20 % (4,140) of them underwent endoscopic esophageal resection. These over 4,000 patients can be considered as potential recipients of cell-sheet treatments. A large number of esophageal cancer patients are male, and the disease prevalence rate is on the rise. Given that the growth in the number of deaths is lower than that of the disease prevalence rate, the demand for treatments for post esophageal cancer surgery and esophageal narrowing is estimated to be on the rise.
 Cell Processing Center: CellSeed (CPC) Installation Being Considered
(2) Regenerative Medicine Support Business
Opportunities to secure new earnings sources will be expanded along with the development of new product and new sales partners. In addition to the fortification of temperature-responsive cell cultureware products as a part of new product development efforts, the product development of clinical applications for cell-sheet transplant devices is also being considered. Moreover, efforts will be focused upon development of a diverse range of companies and research institutions that handle cells as a part of CellSeed's strategy of cultivating new customers.
<Capital Policy>
CellSeed has adequate funding to execute its mid-termmid-termmid-term business plan as evidenced by the approximately ¥3.0 billion in cash and equivalents on hand at the start of fiscal year December 2014. However, the Company is also considering further capital procurement from the perspective of the mid to long term (Capital procurement is expected to be implemented within the framework of this mid-termmid-termmid-term business plan).
|
||||||||||||
| Conclusions |
|
At the same time, the strategic positioning of the regenerative medicine support business within CellSeed has changed dramatically. Until now, the cultivation and support of collaborating research institutions had been given higher priority than profit contribution regarding the sales of temperature-responsive cell cultureware. However, the potential of temperature-responsive cell cultureware is large, given the potential growth in the research market related to cells and regenerative medicine andits functionality are at the forefront of the world. Consequently, sales of these products will be aggressively pursued. In addition to the ability to acquire earnings opportunities in the short-term, sales of temperature-responsive cell cultureware is expected to contribute to stable long-term earnings and to support CellSeed's management foundation. This report is created based upon information obtained from the results briefing and interview with President and CEO Setsuko Hashimoto. After the interviews were conducted, Hashimoto has made an announcement asking "for further patience to shareholders." While CellSeed has been forced to drastically revise its earnings projections due to changes in the approval process for regenerative medicines in Europe, Hashimoto's explanation of the current strategy of cultivating the seeds sewn and the current direction of the Company's strategy was convincing. Furthermore, Prime Minister Abe's policies have placed a top priority upon cultivating the regenerative medicine applications. Therefore, the strategy of cultivating the seeds sewn appears to be justified. The start of clinical trials in Japan and Sweden during the second half of fiscal year December 2015 and preparations for the start of clinical trials of regenerated cartilage sheet in fiscal year December 2016 are considered to be the first two milestones and should be watched closely. Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2015 Investment Bridge Co., Ltd. All Rights Reserved. |




