Bridge Report: (7776) CellSeed
 Setsuko Hashimoto, President &CEO | CellSeed Inc.(7776) |
 |
Company Information
Exchange | JASDAQ |
Industry | Precision Instrument (Manufacturing) |
President | Setsuko Hashimoto, Ph.D. |
HQ Address | Telecom Center Building, Aomi 2-5-10, Koto-ku, Tokyo |
Year-end | December |
Homepage |
Stock Information
Share Price | Shares Outstanding | Market Cap. | ROE (Actual) | Trading Unit | |
¥784 | 11,459,265 shares | ¥8,984 million | 9.8% | 100 shares | |
DPS (Est.) | Dividend Yield (Est.) | EPS (Est.) | PER (Est.) | BPS (Actual) | PBR (Actual) |
- | - | - | - | ¥121.19 | 6.5 x |
* Stock price as of closing on February 27, 2019. Number of shares at the end of the most recent quarter excluding treasury shares.
ROE and BPS are the actual values of the previous term.
Earnings Trends
Fiscal Year | Sales | Operating Profit | Current Profit | Net Profit | EPS | DPS |
December 2015 | 193 | -568 | -531 | -535 | - | - |
December 2016 | 100 | -1,413 | -1,415 | -1,414 | - | - |
December 2017 | 85 | -956 | -964 | -966 | - | - |
December 2018 | 1,026 | 140 | 140 | 129 | 11.35 | - |
December 2018 Est. | 300 | -1,100 | -1,100 | -1,100 | - | - |
* Estimates are those of the company. From FY12/16, the definition of net income has been changed to net income attributable to the parent company shareholders (the same shall apply hereinafter).
This Bridge Report presents the fiscal year 2018 earnings results and the future outlook of CellSeed Inc.
Table of Contents
Key Points
1. Company Overview
2. Fiscal Year 2018 Earnings Results and Estimates
3. Mid-term Business Plan (Fiscal Year 2019 to 2021)
4. Future Highlights
Reference: Regarding Corporate Governance
Key Points
- For fiscal 2018, sales were 1,026 million yen (85 million yen in the previous year) and operating income was 140 million yen (an operating loss of 956 million yen in the previous term). In the cell sheet regenerative medicine business, the company received 960 million yen for technological transfer and other projects from its collaborative business partner in Taiwan according to the progress in development. As the investment in development was recouped, CellSeed successfully went into the black for the first time since its establishment, which means the company did not meet the condition for “Profits” of the Criteria for Delisting set forth by the Japan Exchange Group and managed to avoid satisfying the condition for “Corporate Performance” of the same criteria for the time being.
- CellSeed completed clinical trials of the epithelial cell sheet for esophageal regeneration in the first quarter of fiscal 2019. CellSeed received a feedback from the Pharmaceuticals and Medical Devices Agency (PMDA), which is a certifying body, stating that the safety of the epithelial cell sheet has been confirmed, but it is not certain that the data obtained through the trials about its efficacy is detailed enough, and thus, CellSeed needs to conduct additional clinical trials and submit data that support the efficacy before applying for approval for manufacturing and sale. CellSeed aims to submit a clinical trial notification for another trial by the end of fiscal 2019, complete the additional clinical trial in fiscal 2021, and apply for manufacturing and sale approval in fiscal 2022. Therefore, the company is expected to continue to make upfront investment for some time to come.
- Although the results of the clinical trials of the epithelial cell sheet for esophageal regeneration were unfortunate, President & CEO Hashimoto made a comment as follows: “We gained knowledge and insight through the trial, which we would not otherwise have been able to glean. The clinical trials enabled our company to recognize that we must deliver this product to patients as soon as we can. We would like to make the best use of these thoughts and experiences for planning the next clinical trial. Our company will continue discussing with PMDA and forging ahead with product development for the purpose of providing patients with this therapeutic approach without further delay.” We would like to expectantly await the company’s future progress.
1. Company Overview
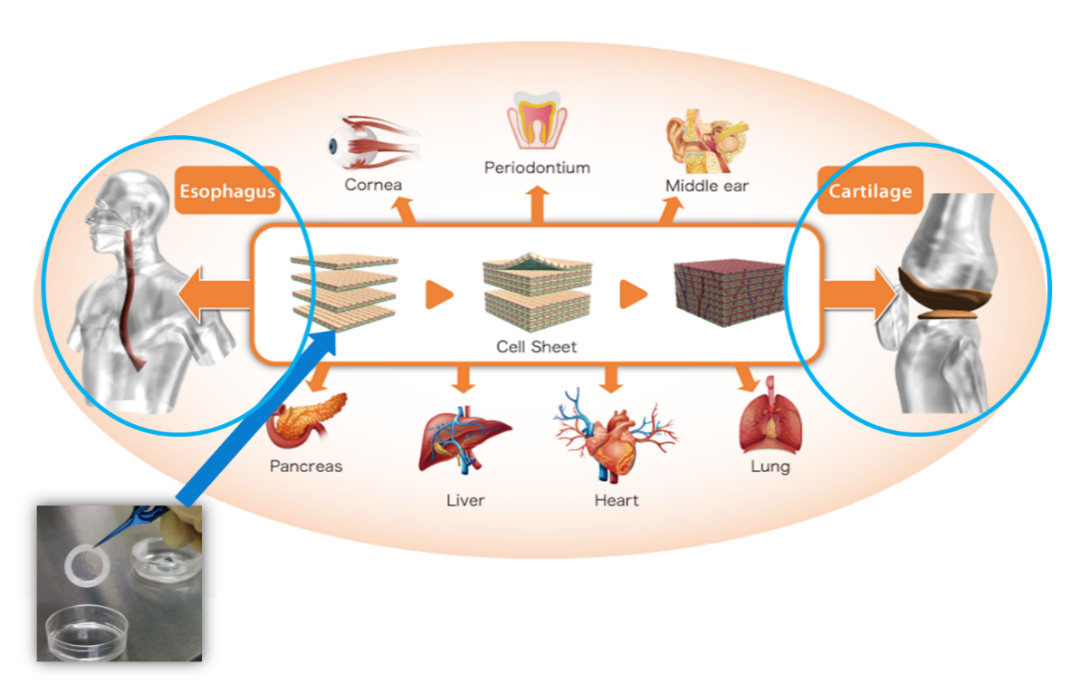
CellSeed uses the fundamental technologies of “cell sheet engineering” developed in Japan by Professor Okano of the Tokyo Women’s Medical University in its “cell sheet regenerative medicine” that employs “cell sheets” for the cell regenerative medicine business, and the regenerative medicine support business, where temperature responsive cell cultureware used to fabricate cell sheets are developed and sold and the regenerative medicine consignment services, which support for research and development and commercialization of regenerative medicine, is provided.
1-1 Cell sheet regenerative medicine business
Commercialization through the cultivation of new businesses is being conducted in joint clinical research efforts with universities. The current development pipeline consists of the two main realms of “epithelial cell sheet for esophageal regeneration” based upon the fundamental technology of “regenerative cell sheet engineering”, and “regenerated cartilage sheet” of knee osteoarthritis.
A clinical trial of the “epithelial cell sheet for esophageal regeneration” was completed in the first quarter of fiscal 2019 in Japan; however, the company has been required to perform another clinical test, and thus it will submit a clinical trial notification for the additional trial during fiscal 2019 with the aim of applying for approval for manufacturing and sale in fiscal 2022. As for business in overseas nations, CellSeed entered into a business alliance with MetaTech (AP) Inc. (hereinafter referred to as MetaTech) in Taiwan in April of fiscal 2017 and the company submitted a clinical trial notification at the end of December 2018.
Meanwhile, the “regenerated cartilage sheet,” for which Tokai University Hospital had submitted an application for approval, was approved as advanced medicine in January 2019, allowing the company to prepare for starting to offer the medical treatment at the university hospital. Furthermore, CellSeed has licensed out the product to MetaTech, and MetaTech is putting forth efforts to commercialize it in Taiwan. Application of regenerative medicine products using the “cell sheet engineering” technology is not limited to the esophagus or knee cartilage. Clinical research is being propelled forward with regard to treatment of other tissues, including the cornea, periodontium, middle ear, lung, heart, liver, and pancreas, and clinical data are already available for these applications. The company is now considering and selecting the third development pipeline and geographic region and will embark on product development once all the necessary arrangements have been made, such as contracts with research institutions.
(Source: CellSeed)

UpCell® (Cell Sheet Recovery Use)
Temperature-responsive cell cultureware for "Cell Sheet" engineering

RepCellTM (Cell Recovery Use)
Temperature-responsive cell cutureware for cell collection

HydroCellTM
The Ultralow Adhesion Cell Cultureware
(All of the pictures above are taken from CellSeed’s website.)
1-2 Regenerative medicine supporting business
Consigned regenerative medicine services for the comprehensive support regarding regenerative medicine and development, manufacture, and sales of temperature responsive cell cultureware are being conducted. The main services provided within the consigned regenerative medicine services include regenerative cell sheet product manufacturing method development, consigned manufacturing, operational and application support, cell culturing technician training and others.
1-2-1 Development of Manufacturing Methods for Cell Sheet Products and Contract Manufacturing
CellSeed develops and manufactures mainly cell sheet products on consignment from pharmaceutical companies and research institutions. The company has hired clinical cultivatists certified by the Japanese Society for Regenerative Medicine, and its staff with extensive experience in cell culture develop manufacturing methods for regenerative medicine products using high-quality cell sheet, etc. and manufacture such products at the Cell Processing Facility (CPF) approved for manufacturing and processing specified cell products.
The company has been moving ahead with research and development of the regenerated cartilage sheet in the cell sheet regenerative medicine business, and the product, for which Tokai University had submitted an application for approval, was approved in January 2019 as advanced medicine. Following the approval, arrangements for a clinical trial are being made. It has been decided that CellSeed will culture (process) the cell sheet, which will be used in the aforementioned application of advanced medicine, at the Cell Processing Facility on consignment from Tokai University.
“The Act on Safety of Regenerative Medicine” stipulates operation of buildings and facilities, and cell processing, requiring submitting notification and approval application to the Minister of Health, Labour and Welfare for each Cell Processing Facility. CellSeed provides support services of submission of notification and preparation of application forms for manufacturing and processing specified cell products, and preparation of documents necessary for operation. It also assists clients with administrative procedures required, such as preparation of documents for consultation with the authorities concerned that will be necessary for submitting application, including clinical trial notification, and preparation of application forms.
1-2-2 Training and Education in Cell Culturing Technology
CellSeed trains and educates cell culturing engineers who have little or no experience in manufacturing or processing cell sheets about various matters, including the procedures for culturing and exfoliating cell sheets.
1-2-3 Cell Processing Facility (CPF)
This Cell Processing Facility boasts of 763 square meters of floor space, and has an automated monitoring system to control cleanliness, room pressure, temperature and humidity, Operational status of equipment (Incubator, Reagent stocker etc.) and a surveillance camera system throughout the entire facility. In addition, this facility is only 20 minutes drive from Haneda International Airport. In March 2017, “manufacture and process specified cell products ” in accordance with Article 35, paragraph 1 of the Act on Safety of Regenerative Medicine was granted by the Ministry of Health, Labour and Welfare. Consequently, CellSeed is able to provide consigned processing business for cell sheets.

2. Fiscal Year 2018 Earnings Results and Full-Year Estimates
2-1 Consolidated Earnings
| FY Dec. 17 | Share | FY Dec. 18 | Share | YY Change | Initial Est. | Divergence |
Sales | 85 | 100.0% | 1,026 | 100.0% | - | 1,170 | -12.3% |
Gross Income | 51 | 60.0% | 994 | 96.9% | - | - | - |
SG&A | 1,007 | - | 854 | 83.2% | -15.2% | - | - |
Operating Income | -956 | - | 140 | 13.6% | - | 20 | +600.0% |
Ordinary Income | -964 | - | 140 | 13.6% | - | 50 | +180.0% |
Net Income | -966 | - | 129 | 12.6% | - | 40 | +222.5% |
※ Figures include reference figures calculated by Investment Bridge Co., Ltd. Actual results may differ (the same applies to all tables below).
Unit: ¥mn
Change in the Accounting Policy
Conventionally, the company recorded subsidies related to research and development of regenerative medicine under “Subsidy Income” of non-operating income; however, it made a change and started to deduct such subsides from “Research and Development Expenses” of selling, general and administrative (SG&A) expenses in fiscal 2018 with the aim of clarifying the actual conditions of the costs incurred by CellSeed and presenting the profit and loss section in a more appropriate manner. In order to make it possible to draw a comparison with fiscal 2018, the SG&A expenses and operating loss were accounted for retroactively for fiscal 2017.
Sales were 1,026 million yen (85 million yen in the previous term), and operating income was 140 million yen (an operating loss of 956 million yen in the previous term).
Although sales in the regenerative medicine supporting business were 66 million yen, nearly unchanged from the previous term, the company posted sales of 960 million yen in the cell sheet regenerative medicine business, which is attributed to the exclusive business alliance concluded in Taiwan. Amid a significant increase in gross income, research and development expenses dropped from 564 million yen in the previous term to 432 million yen, and other SG&A expenses shrank from 443 million yen of last year to 421 million yen, which resulted in a considerable profit rise. Sales fell short of the initial forecast, because the company did not enter into any new business alliance or licensing contract. Each of the incomes considerably exceeded the initial estimates due to a number of factors, such as the expenses for development outsourcing and costs for maintaining the Cell Processing Facility that came short of their respective initial forecasts.
Sales and Operating Income by Segment
| FY12/17 | Share | FY12/18 | Share | YY Change |
Regenerative medicine supporting business | 69 | 81.5% | 66 | 6.4% | -4.7% |
Cell sheet regenerative medicine business | 15 | 18.5% | 960 | 93.6% | - |
Sales, Total | 85 | 100.0% | 1,026 | 100.0% | - |
Regenerative medicine supporting business | -98 | - | -70 | - | - |
Cell sheet regenerative medicine business | -547 | - | 497 | - | - |
Adjustments | -311 | - | -287 | - | - |
Operating Income, Total | -956 | - | 140 | - | - |
Unit: ¥mn
2-2 Financial condition and Cash flow
Financial Conditions
| Dec. 17 | Dec. 18 |
| Dec. 17 | Dec. 18 |
Cash | 1,350 | 1,057 | Accounts payable | 107 | 56 |
Current Assets | 1,477 | 1,505 | Advances received | 148 | 64 |
Tangible Assets | 21 | 19 | Liabilities | 303 | 174 |
Investments, Others | 61 | 61 | Net Assets | 1,258 | 1,411 |
Noncurrent Assets | 84 | 81 | Total Liabilities, Net Assets | 1,561 | 1,586 |
Unit: ¥mn
Summarized Cash Flow Statement
| FY12/17 | FY12/18 | YYChange | |
Operating Cash Flow | -747 | -306 | +440 | - |
Investing Cash Flow | -5 | -1 | +3 | - |
Financing Cash Flow | 1,040 | 24 | -1,016 | -97.6% |
Cash, Equivalents at Term End | 1,350 | 1,057 | -292 | -21.7% |
Unit: ¥mn
3. Medium Term Business Plan (Fiscal Years December 2019 to 2021)
<Key Points>
●Apply the manufacturing and sales approval for epithelial cell sheet for esophageal regeneration
● Accelerate development of regenerated cartilage sheet
● Launch the development of the next products following the regenerated esophageal sheet and the regenerated cartilage sheet
● Establish a supply chain system
● Develop new regenerative medicine supporting products and cultivate earnings opportunities
● Promote business alliance for global expansion
The company aims to apply for approval for manufacturing and sale of the epithelial cell sheet for esophageal regeneration at an early stage, and propel forward preparation for clinical trials of the regenerated autologous cartilage sheet and accelerate development of the regenerated autologous (allogeneic) cartilage sheet. Furthermore, it will begin to develop the next product following the epithelial cell sheet for esophageal regeneration and the regenerated cartilage sheet, and strive to build an organizational structure and infrastructure. In the regenerative medicine supporting business, the company will move ahead with development of new regenerative medicine supporting products and endeavor to seize more opportunities for raising revenue in the consignment services business. CellSeed will proactively forge business alliances with overseas firms for the purpose of globally expanding the cell sheet engineering as the technology originally developed in Japan.
【Efforts to Achieve Targets of Mid-term Business Plan】
Application for Approval for Manufacturing and Sale of the Epithelial Cell Sheet for Esophageal Regeneration
According to CellSeed, 22,000 patients within Japan are diagnosed with esophageal cancer every year (90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma), with 11,500 patients dying every year. In addition, the rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients, with five year survival rates for males and females said to be 36% and 44%, respectively. The endoscopic resection surgery (ESD) was posted in the drug price list from 2008 and is on the rise, but its side effect of esophageal stricture after surgery has been recognized as a problem.
The company strives to put the epithelial cell sheet for esophageal regeneration into practice, aiming to improve patients’ quality of life by reducing the frequency of occurrence of esophageal strictures.
(Source: CellSeed)
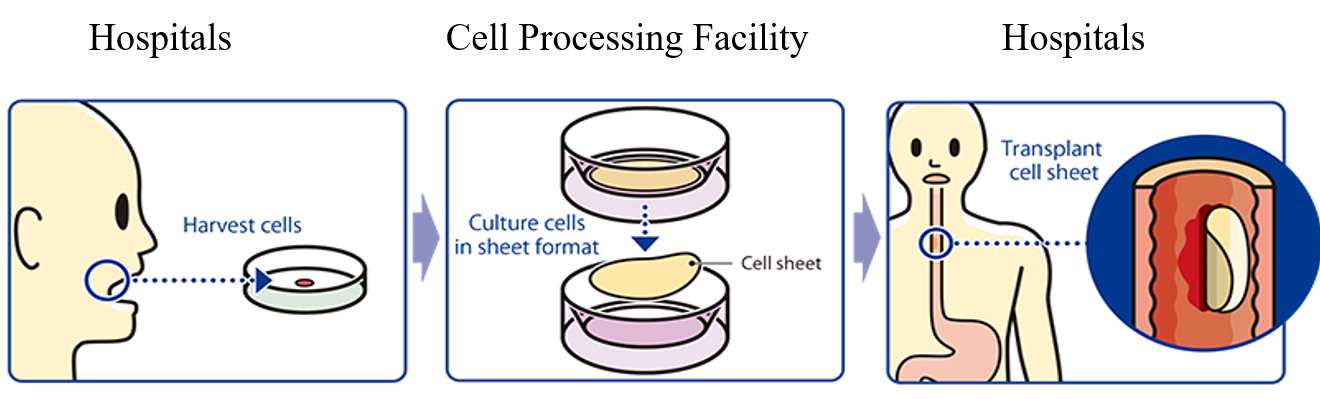
The medical treatment using the “epithelial cell sheet for esophageal regeneration” is a regenerative medical treatment method for esophageal cancer (for healing esophageal tears and preventing strictures), which has been developed by the Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University. Cells taken from the oral mucosa of a patient are cultured for about 2 weeks using the temperature-responsive cell cultureware to produce cell sheets. In conjunction with the process of culturing cell sheets, an endoscopic surgery for esophageal cancer excision is performed and the cell sheets are transplanted to the part of an esophageal tumor in the patient.
There have been 30 clinical cases from 2008 to 2014, 10 cases at TWMU, 10 cases at TWMU and Nagasaki University (Long-distance transportation test: Collect cells at Nagasaki University, Culture at TWMU, Transplant at Nagasaki University), and 10 cases at Karolinska University Hospital (Sweden). CellSeed has concluded Basic Agreement for Development with TWMU and has taken over the research results.
In Japan, following the submission of a clinical trial notification in the second quarter (April) of fiscal 2016, the company launched clinical trials at the National Cancer Center Japan (National Cancer Center Hospital and National Cancer Center Hospital East) and Tokyo Women’s Medical University in the third quarter (August) of the same term. The company finished with case registration in the second quarter of fiscal 2018 and completed the clinical trials in the first quarter of fiscal 2019.
In overseas countries, it set up a subsidiary, CellSeed Sweden AB, in Sweden in the second quarter (May) of fiscal 2015 and held a preliminary consultation with the Swedish Medical Product Agency (MPA) in the fourth quarter (November) of the same year. It formed a business alliance with MetaTech in Taiwan in the first quarter (April) of fiscal 2017, and following that, the company submitted a clinical trial notification at the end of December 2018.
Overview of the Clinical Trial Results
The company will conduct another clinical trial because reliable data on the efficacy were not amassed.
No side effect was reported in the clinical trials, meaning that no safety issues were confirmed; on the other hand, the efficacy rate of the “effectiveness of stricture prevention 8 weeks after endoscopic submucosal dissection (ESD)” (rate of cases of non-stenosis), which was an important evaluation item, was only 12.5%, which did not prove the statistical superiority to the threshold response rate (the rate of cases of non-stenosis in patients who did not receive any treatment after ESD).
Regarding the results of the aforementioned clinical trials, while working on confirming the data, the company had been discussing with the Pharmaceuticals and Medical Devices Agency (PMDA), which is a certifying body, through regular interviews; however, in February 2019, CellSeed received a feedback from PMDA, stating that the safety of the product has been confirmed, but it is not certain that the data obtained through the trials are detailed enough about its efficacy, and thus, CellSeed needs to conduct additional clinical trials and submit data that supports the efficacy before applying for approval for manufacturing and sale. Based on the feedback, the company plans to continue holding discussion with PMDA to carry out additional clinical trials. It aims to submit a clinical trial notification for another trial by the end of fiscal 2019, complete the additional clinical trial in fiscal 2021, and apply for manufacturing and sale approval in fiscal 2022.
(Source: CellSeed)
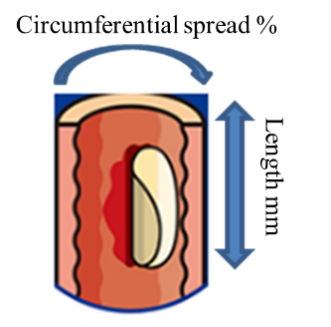
Ten cases were registered, and 2 cases out of the 10 cases were deregistered, resulting in 8 registered cases as it turned out, but the efficacy was confirmed only for one of the cases. The clinical trials were performed in accordance with inclusion criteria, in other words, standards for the excision size, which were the circumferential spread of the major lesion of over 75% and the length of the major lesion of less than 80 mm; however, only 2 of the 8 successfully registered cases satisfied the criteria, and the actual excision sizes in 6 of the registered cases were longer than 80 mm, exceeding the initial inclusion criteria targets. According to the company, this is one of the most challenging elements in clinical trials of regenerative medicine products. When the excision size is larger, the rate of stricture occurrence is higher. For your information, when the clinical research and clinical trials conducted are taken into account together, the efficacy of the cases that have met the inclusion criteria is 57.1% (the efficacy has been confirmed in 8 cases of a total of 14 cases).
The company accelerates development of the regenerated cartilage sheet in a bid to launch clinical trials.
Collaborative Research with Tokai University
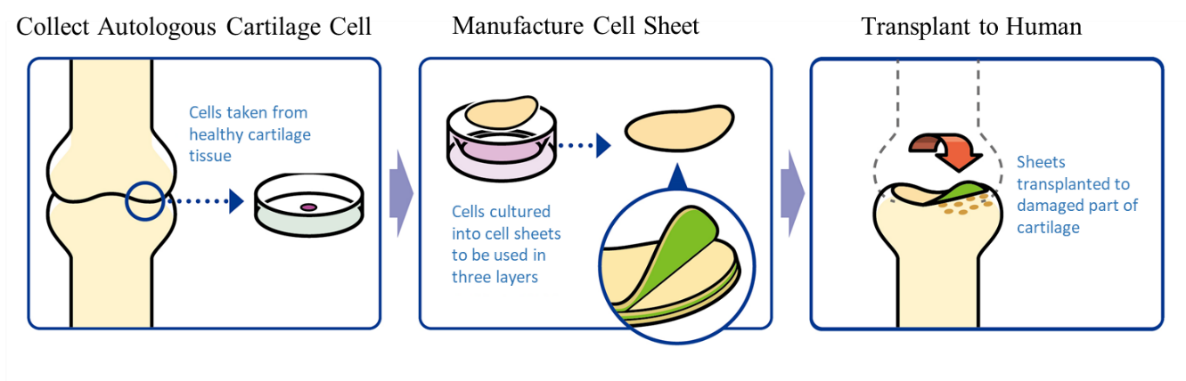
The company started providing the temperature-responsive cell cultureware in 2004 and began collaborative research with Tokai University in 2006. It began to support the university’s clinical research in 2010 and dispatch researchers in 2014, strengthening partnership with Tokai University in collaborative research. Clinical research into the transplant of regenerated cell sheets was approved in August 2011 at the 65th meeting of the Committee on Scientific Technology of the Health Sciences Council, and a written opinion of the Minister of Health, Labour and Welfare (issued by the Health Policy Bureau of the Ministry of Health, Labour and Welfare, No. 1003-3) was published in October of the same year. The first clinical trial was launched in November of the year, and the company completed follow-up assessments, after 2 years, of 8 cases in November 2015. In addition, a “cartilage treatment method through cartilage regeneration using regenerated cell sheets,” for which Tokai University Hospital had submitted an application for approval, was approved as advanced medicine by the Ministry of Health, Labour and Welfare at the “71st advanced medical care meeting” in January 2019. CellSeed will manufacture the regenerated cartilage sheet on consignment from the university.
CellSeed cooperates with Tokai University in research and development, which is aimed to explore factors in the efficacy of the cartilage cell sheet and realize medical treatment using the regenerated autologous cartilage sheet for treating knee osteoarthritis, through the “Project Focused on Development of Key Evaluation Technology: Evaluation for Industrialization in the Field of Regenerative Medicine (support for accelerating development of technological seeds in the field of regenerative medicine),” a subsidy program offered in fiscal 2018 by the Japan Agency for Medical Research and Development (AMED). In addition, it also participated in AMED’s project of developing “comprehensive evaluation methods for the efficacy of autologous cartilage cell sheets” together with DNA Chip Research Inc.
Indication:deformed cartilage and osteoarthritis (Source: CellSeed)
Development of Methods for Autologous Cell Sheet Transplant
The world’s first clinical research into the transplant of autologous cell sheets was carried out on February 15, 2017 (a transplant operation took place). This clinical research took cartilage tissues from polydactyly patients, cultured cell sheets for 2-3 weeks, and then transplanted the sheets (with consent secured to use cartilage tissues of the fingers of infants who congenitally had 6 fingers). The company plans cell sheet transplants to 10 patients for the next 3 years and will complete the transplant projects by March 2020. At the same time, the company embarked on establishing a cell bank and automating cell sheet manufacturing. It is scheduled to begin a corporate clinical trial in 2021 with AMED’s support.
Regenerative Medicine Consignment Services
The company provides a wide range of regenerative medicine services on consignment in relation to cell sheet products, including development of manufacturing methods and contract manufacturing, facility management and application support, and training and education in cell culturing technology.
The company engages in manufacturing on consignment and quality testing for cell sheet products as services of development of manufacturing methods and contract manufacturing for cell sheet products. These services are characterized by a number of staff members with extensive knowledge and experience with cell culturing practices, such as clinical cultivatists certified by the Japanese Society for Regenerative Medicine, and production of cell sheets using the company’s product UpCell® at the facility with permission for manufacturing and processing specified cell products (Facility Number.: FA3160008). CellSeed received an order in November 2018 for a project related to manufacturing of the mesenchymal stem cell sheet derived from the autologous periodontal membrane used in a research project for industrializing regenerative medicine, “Periodontal Tissue Regenerative Therapy with Periodontal Ligament-Derived Mesenchymal Stem Cell Sheets,” by the Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University (with Professor Takanori Iwata as the person in charge of this research and development project), which was adopted by AMED.
The company also offers facility management and application support services, such as preparation of application forms for manufacturing and processing specified cell products, application and submission, consulting for document preparation, maintenance of facility equipment and management systems, and management support. The training and education in cell culturing technology include training programs regarding culturing and exfoliation of cell sheets.
Consideration regarding the Next Development Pipeline
The company will determine the third item to develop following the epithelial cell sheet for esophageal regeneration (Japan) and the regenerated cartilage sheet (Japan), and start commercializing the product by the end of 2020. It will begin specifically considering a development pipeline and geographic region as soon as all arrangements have been made, including a contract with an institution that conducts the research.
Promotion of Business Alliances toward Global Business Expansion
The company will propel forward support for MetaTech that is an existing business partner in Taiwan. CellSeed plans to cooperate with MetaTech in setting up a new research and development base under the Taiwanese government. It will also strive to conclude contracts with other firms. Specifically, the company will continue business activities mainly in Asian nations, Europe, and the United States in an attempt to enter into business alliance and licensing contracts (although some companies, like MetaTech, express interest in CellSeed’s projects under development, CellSeed did not conclude any contract in fiscal 2018). In conjunction with the effort to improve its business value by promoting development of the pipelines which are in process, the company aims to prospect for potential companies to build partnerships with during the period of the mid-term business plan, targeting enterprises in Asian countries, Europe, and the United States.
CellSeed created a business alliance with MetaTech in April 2017, and granted MetaTech exclusive rights to development, manufacturing, and sale in Taiwan for its cell sheet regenerative medicine business (the epithelial cell sheet for esophageal regeneration and the regenerated cartilage sheet). MetaTech moves ahead with development and commercialization of the products in Taiwan with support from CellSeed. CellSeed received 960 million yen for technological transfer and other projects in fiscal 2018, according to the progress with development.
【numerical target】
| Dec. 19 Est. | Dec. 20(goal) | Dec. 21(goal) |
Regenerative medicine supporting business | 150 | 225 | 300 |
Cell sheet regenerative medicine business | 150 | 125 | 1,700 |
Sales | 300 | 350 | 2,000 |
Operating Income | -1,100 | -1,300 | 300 |
Ordinary Income | -1,100 | -1,300 | 300 |
Net Income | -1,100 | -1,300 | 225 |
Unit: ¥mn
The regenerative medicine supporting business is expected to show steady growth thanks to the first order that the company received in November 2018 in the regenerative medicine consignment services; however, CellSeed as a whole is projected to continue making upfront investment in fiscal 2019 and 2020. The company is expected to seek opportunities for business alliances in the cell sheet regenerative medicine business to earn revenue in fiscal 2021.
Event Hosted by CellSeed
CellSeed will host the first cell sheet engineering innovation forum on July 19, 2019 (13:30-18:00) at the Tokyo Metropolitan Industrial Technology Research Institute with seats available for 150 people (the forum is designed exclusively for researchers; participants are required to register in advance and seats are served on a first-come-first-served basis).
The following professors are scheduled to give lectures: Professor Emeritus TeruoOkano at Tokyo Women’s Medical University; Director Tatsuya Shimizu at the Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University; Professor Masato Sato at Department of Orthopaedic Surgery, Tokai University School of Medicine; and Professor Takanori Iwata, the head of Faculty of Periodontology at Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University.
The company is inviting slogan ideas for the poster for research projects using the “cell sheet” or “temperature-responsive cell cultureware.”
4. Future Highlights
Although the results of the clinical trial of the epithelial cell sheet for esophageal regeneration were unfortunate, President & CEO Hashimoto made a comment as follows: “We gained knowledge and insight through the trials, which we would not otherwise have been able to glean. The clinical trials enabled our company to recognize that we must deliver this product to patients as soon as we can. We would like to make the best use of these thoughts and experiences for planning the next clinical trial. Our company will continue discussing with PMDA and forging ahead with product development for the purpose of providing patients with this therapeutic approach without further delay.” Meanwhile, the regenerated cartilage sheet, for which Tokai University Hospital had submitted an application for approval, was approved in January 2019 as advanced medicine, allowing the company to propel forward preparations to start providing medical treatment using the product at the university hospital. CellSeed will continue putting forth efforts in unison to realize and commercialize regenerative medicine as soon as possible. We would like to expectantly await the company’s future progress.
Reference: CellSeed’s corporate governance
◎ Organization type, and the composition of executive directors and auditors
Organization type | Company with company audAitor(s) |
Executive Directors | 4 directors, including 2 external ones |
Corporate Auditors | 3 corporate auditors, including 2 external ones |
◎Corporate Governance Report
Latest Update: April 6, 2018
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people’s health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.
In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)>
CellSeed has stated, “Our company implements all the basic principles stipulated in the Corporate Governance Code as a JASDAQ listed company.”
This report is intended solely for information purposes, and is not intended as a solicitation for investment. The information and opinions contained within this report are made by our company based on data made publicly available, and the information within this report comes from sources that we judge to be reliable. However, we cannot wholly guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration. Copyright(C) 2019 Investment Bridge Co., Ltd. All Rights Reserved. |
To view back numbers of Bridge Reports on CellSeed (7776) and other companies, or IR related seminars of Bridge Salon, please go to our website at the following url.
URL:https://www.bridge-salon.jp/


