Bridge Report:(7776)CellSeed the fiscal year December 2019
 Setsuko Hashimoto, President &CEO | CellSeed Inc.(7776) |
 |
Company Information
Market | JASDAQ |
Industry | Precision Instrument (Manufacturing) |
President | Setsuko Hashimoto, Ph.D. |
HQ Address | Telecom Center Building, Aomi 2-5-10, Koto-ku, Tokyo |
Year-end | December |
Homepage |
Stock Information
Share Price | Number of shares issued (excluding treasury shares) | Total market cap | ROE Act. | Trading Unit | |
¥326 | 12,981,665shares | ¥4,232million | - | 100 shares | |
DPS Est. | Dividend yield Est. | EPS Est. | PER Est. | BPS Act. | PBR Act. |
- | - | - | - | ¥102.24 | 3.2x |
*Stock price as of closing on February 28, 2020. Number of shares at the end of the most recent quarter excluding treasury shares. ROE and BPS are the actual values of the previous term.
Earnings Trend
Fiscal Year | Sales | Operating Profit | Current Profit | Net Profit | EPS | DPS |
December 2016 | 100 | -1,413 | -1,415 | -1,414 | - | - |
December 2017 | 85 | -956 | -964 | -966 | - | - |
December 2018 | 1,026 | 140 | 140 | 129 | 11.35 | - |
December 2019 | 275 | -780 | -786 | -782 | - | - |
December 2020 Est. | 310 | -1,020 | -1,020 | -1,020 | - | - |
* Estimates are those of the company (Unit: million yen, yen).
This Bridge Report presents the fiscal year ended December 2019 earnings results and the future outlook of CellSeed Inc.
Table of Contents
Key Points
1. Company Overview
2. Fiscal Year ended December 2019 Earnings Results
3. Trends by segment
4. Mid-term Business Plan(FY 12/20 to FY 12/22)
5. Conclusions
<Reference: Regarding Corporate Governance>
Key Points
- For the term ended December 2019, sales dropped 73.1% year on year, and operating loss was 780 million yen (an operating income of 140 million yen was posted in the previous term). While the sales in the regenerative medicine supporting business increased 77.2% year on year, the sales in the cell sheet regenerative medicine business decreased 83.5% year on year due to the decreased sales based on the contract on exclusive business alliance in Taiwan, however, the results were in line with the forecast made at the beginning of the term for the most part. The decline in sales resulted in an operating loss, but they largely exceeded the initial forecast as the expenses for outsourcing for development and maintaining Cell Processing Facility were below the expected levels.
- The regenerative medicine service began to contribute to earnings with “periodontal ligament cell sheets,” while the sales of equipment marked a record high. Its sales grew mainly outside Japan. The sales of equipment will contribute not only to earnings, but also to increased recognition of “cell regenerative sheets,” which use fundamental technologies of “cell sheet engineering.” As for the business in Taiwan, “transplantation of autologous cartilage cells” applied by MetaTech’s partner hospital E-Da Hospital at the end of December last year, was approved as advanced medicine. The joint venture established in Taiwan has been blessed with a number of partners and is expected to expand in the future.
- On the other hand, “epithelial cell sheet for esophageal regeneration” needs to be targeted at patients at risk for steroid administration, and therefore, discussions have been going on with the PMDA regarding the target patients and the number of cases. The widespread use of inexpensive steroid therapy to prevent stenosis after endoscopic treatment of esophageal cancer is likely to influence the matter. Not all of things are in the favor, but there is no concern about the company’s financial base. We would like to pay attention to how much the company is able to grow and increase the number of positive factors in the fiscal year ending December 2020.
1. Company Overview
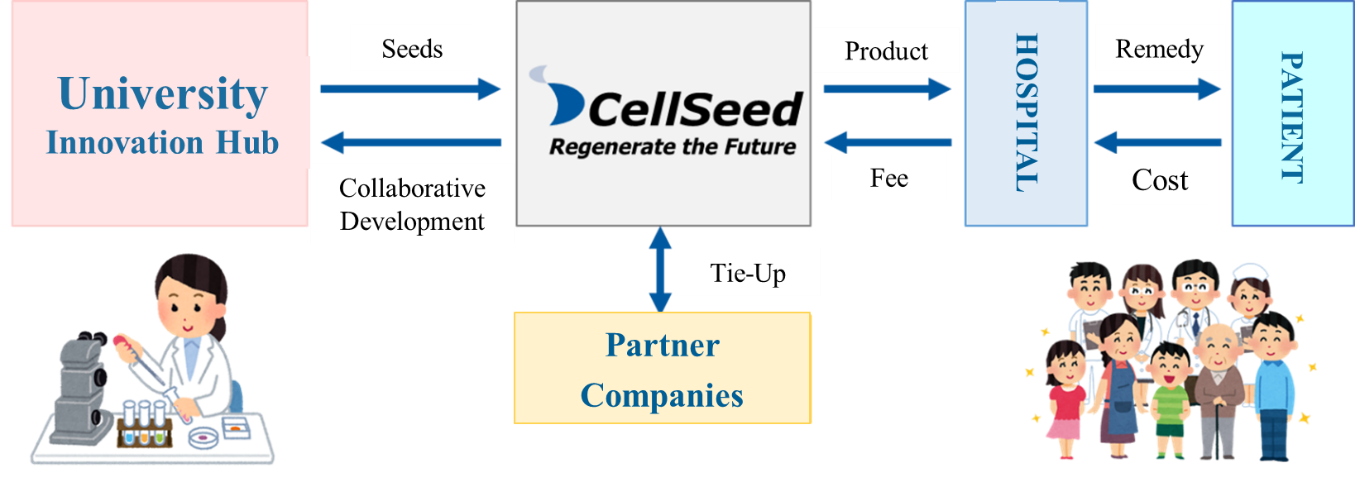
Regenerative medicine is a new kind of medicine for regenerating and curing lost, damaged or deteriorated tissues. CellSeed uses the fundamental technologies of “cell sheet engineering” developed in Japan by Professor Okano of the Tokyo Women’s Medical University in its “cell sheet regenerative medicine” that employs “cell sheets” for the cell regenerative medicine business, and the regenerative medicine support business, where temperature responsive cell cultureware used to fabricate cell sheets are developed and sold and the regenerative medicine consignment services, which support for research and development and commercialization of regenerative medicine, is provided.
Mission: We take the initiative of contributing to global health care in the valuable and innovative field of regenerative medicine.
Using the outcomes of research at universities as seeds, the company conducts clinical trials and develop regenerative medicine products, with the aim of contributing to medicine around the world.
(Source: the company)
1-1.Business Description
Cell sheet regenerative medicine business
Commercialization through the cultivation of new businesses is being conducted in joint clinical research efforts with universities. The current development pipeline consists of the two main realms of “epithelial cell sheet for esophageal regeneration” based upon the fundamental technology of “regenerative cell sheet engineering”, and “regenerated cartilage sheet” of knee osteoarthritis.
The clinical trial for “epithelial cell sheet for esophageal regeneration” was completed in the first quarter of the term ended December 2019, but the company is currently discussing with the PMDA regarding the required additional clinical trial. As for business in overseas nations, CellSeed entered into a business alliance with MetaTech (AP) Inc. (hereinafter referred to as MetaTech) in Taiwan in April of fiscal 2017 and the company submitted a clinical trial notification at the end of December 2018.
Meanwhile, the “regenerated cartilage sheet,” for which Tokai University Hospital had submitted an application for approval, was approved as advanced medicine in January 2019, allowing the company to prepare for starting to offer the medical treatment at the university hospital. Furthermore, CellSeed has licensed out the product to MetaTech, and MetaTech is putting forth efforts to commercialize it in Taiwan.
Regenerative medicine supporting business
Consigned regenerative medicine services for the comprehensive support regarding regenerative medicine and development, manufacture, and sales of temperature responsive cell cultureware are being conducted. The main services provided within the consigned regenerative medicine services include regenerative cell sheet product manufacturing method development, consigned manufacturing, operational and application support, cell culturing technician training and others.
 | UpCell® Temperature-responsive cell cultureware for collecting cell sheets
|
 | RepCell ™ Temperature-responsive cell cultureware for collecting cells (It collects small cell fragments with the equipped 3 × 3 mm grid wall) |
 | HydroCell ™ The ultralow adhesion cell cultureware |
|
|
(Source: the company)
Development of Manufacturing Methods for Cell Sheet Products and Contract Manufacturing
CellSeed develops and manufactures mainly cell sheet products on consignment from pharmaceutical companies and research institutions. The company has hired clinical cultivatists certified by the Japanese Society for Regenerative Medicine, and its staff with extensive experience in cell culture develop manufacturing methods for regenerative medicine products and manufacture such products at the Cell Processing Facility (CPF) approved for manufacturing and processing specified cell products. As for the regenerated cartilage sheet that is being researched and developed in the cell sheet regenerative medicine business, Tokai University applied for the approval as Advanced Medicine B, and it was approved in January 2019. The cell sheets used for this advanced medicine are scheduled to be cultured at Cell Processing Facility (processing entrusted by Tokai University).
1-2. Cell Processing Facility (CPF)
This Cell Processing Facility boasts of 763 square meters of floor space, and has an automated monitoring system to control cleanliness, room pressure, temperature and humidity, Operational status of equipment (Incubator, Reagent stocker etc.) and a surveillance camera system throughout the entire facility. In addition, this facility is only 20 minutes drive from Haneda International Airport. In March 2017, “manufacture and process specified cell products” in accordance with Article 35, paragraph 1 of the Act on Safety of Regenerative Medicine was granted by the Ministry of Health, Labor and Welfare. Consequently, CellSeed is able to provide consigned processing business for cell sheets.

(Source: the company)
2. Fiscal Year ended December 2019 Earnings Results
2-1 Consolidated Earnings
| FY 12/ 18 | Share | FY 12/ 19 | Share | YY Change | Initial Est. | Divergence |
Sales | 1,026 | 100.0% | 275 | 100.0% | -73.1% | 300 | -8.1% |
Gross Income | 994 | 96.9% | 216 | 78.5% | -78.2% | - | - |
SG&A | 854 | 83.3% | 997 | 362.5% | +16.7% | - | - |
Operating Income | 140 | 13.7% | -780 | - | - | -1,100 | - |
Ordinary Income | 140 | 13.7% | -786 | - | - | -1,100 | - |
Net Income | 129 | 12.6% | -782 | - | - | -1,100 | - |
* unit: million yen
Sales dropped 73.1% year on year, and operating loss was 780 million yen (an operating income of 140 million yen was posted in the previous term)
Sales were 275 million yen, down 73.1% from the previous term when the company achieved significant sales from out-licensing of technology to MetaTech. While the sales in the regenerative medicine supporting business significantly increased 77.2% year on year, sales in the cell sheet regenerative medicine business decreased 83.5% year on year. The company posted an operating loss of 780 million yen. The decline in sales caused the profit of 140 million yen in the previous term to turn into a loss, but the company achieved a significant improvement over the initial forecast as the expenses for outsourcing for development and maintaining Cell Processing Facility were below the expected levels.
2-2 Trends by Segment
| FY12/18 | Share | FY12/19 | Share | YY Change |
Regenerative medicine supporting business | 66 | 6.4% | 117 | 42.5% | +77.2% |
Cell sheet regenerative medicine business | 960 | 93.6% | 158 | 57.5% | -83.5% |
Sales, Total | 1,026 | 100.0% | 275 | 100.0% | -73.1% |
Regenerative medicine supporting business | -70 | - | -46 | - | - |
Cell sheet regenerative medicine business | 497 | - | -424 | - | - |
Adjustments | -287 | - | -310 | - | - |
Operating Income, Total | 140 | - | -780 | - | - |
* Unit: million yen
In the regenerative medicine supporting business, the sales of equipment marked a record high thanks to a substantial increase in overseas sales through participation in numerous trade shows and aggressive sales promotion activities, as well as strategic consolidation of major domestic distributors and sharing of marketing information. Also, in the regenerative medicine business that utilizes the company’s own cell culture center, the company delivered four periodontal ligament cell sheets for investigator-initiated clinical trials entrusted by Tokyo Women’s Medical University. In addition, various activities, such as fibrillation sheet culture training for academia, have resulted in good results.
In the cell sheet regenerative medicine business, on the other hand, sales decreased from 960 million yen in the previous term to 158 million yen, as the company realized sales based on the contract on exclusive business alliance regarding the cell sheet regenerative medicine business with Taiwanese company MetaTech in the previous term.
2-3 Financial condition and Cash flow
Financial Conditions
| Dec. 18 | Dec. 19 |
| Dec. 18 | Dec. 19 |
Cash | 1,057 | 1,065 | Advances received | 64 | 30 |
Receivables | 328 | 56 | Liabilities | 174 | 110 |
Current assets | 1,505 | 1,245 | Net Assets | 1,411 | 1,345 |
Fixed assets | 81 | 210 | Total Liabilities and Net Assets | 1,586 | 1,456 |
* Unit: million yen
Total assets were 1,456 million yen, down 130 million yen from the end of the previous term. Free cash flow was negative, but cash and deposits increased from the end of the previous term due to 718 million yen raised by issuing shares through the exercise of share options.
There is no concern related to the company’s current financial base as it has cash and deposits amounting to 1,065 million yen, but the company has not yet shown a path to early commercialization of the first regenerative medicine product in the cell sheet regenerative medicine business, and therefore, there are circumstances raising questions about the company’s going concern. By promoting the development of epithelial cell sheets for esophageal regeneration and the regenerated cartilage sheets, and through early commercialization of the first cell sheet regenerative medicine product and increase of business partners, the company intends to obtain further earning opportunities and resolve the above situation.
Cash Flow(CF)
| FY12/18 | FY12/19 | YY Change | |
Operating Cash Flow(A) | -306 | -577 | -270 | - |
Investing Cash Flow (B) | -1 | -133 | -132 | - |
Free Cash Flow(A+B) | -308 | -710 | -402 | - |
Financing Cash Flow | 24 | 721 | +696 | - |
Cash and Equivalents at the end of term | 1,057 | 1,065 | +7 | +0.7% |
* Unit: million yen
Operating CF was negative 577 million yen due to a pretax loss of 782 million yen, etc. Investing CF was negative due to the acquisition of investment securities, etc., and financing CF improved due to the proceed from the issuance of shares through the exercise of share options, etc.
2-3 TOPICS
Holding of the cell sheet engineering innovation forum
On July 19, 2019, the company held the first sheet engineering innovation forum at Tokyo Metropolitan Industrial Technology Research Institute (Koto-ku, Tokyo; 135 people in the audience and 23 poster presenters).
The lecturers were Teruo Okano, a professor emeritus of Tokyo Women's Medical University and the director of Cell Sheet Tissue Engineering Center of the University of Utah; Masato Sato, a professor of Department of Orthopaedics, School of Medicine, Tokai University; Takanori Iwata, a senior professor of Department of Periodontology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University; Goshi Shiota, a professor of Division of Molecular and Genetic Medicine, Graduate School of Medical Sciences, Tottori University; and Hidekazu Sekine, a lecturer of Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University.
The themes of posters were research using “cell sheets” or “temperature-responsive cell cultureware.” Mr. Tetsuya Imamura of School of Medicine, Shinshu University received the most excellent poster award, while Mr. Takumi Takahashi of Department of Orthopaedics, School of Medicine, Tokai University and Mr. Tetsutaro Kikuchi of Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University received the excellent poster award. Mr. Tetsuya Imamura of School of Medicine, Shinshu University, who won the most excellent poster award, is engaged in the research on regenerative medicine using mesenchymal stem cells in the field of urology using the Tissue Engineering method.
Plan to hold the second cell sheet engineering innovation forum
On October 15, 2020 (13:30~18:00), the company plans to hold the second cell sheet engineering innovation forum at National Museum of Emerging Science and Innovation (Koto-ku, Tokyo; seating is limited to 200 people and researchers must register in advance). The lecturers are Tatsuya Shimizu, a professor of Tokyo Women’s Medical University. Yuji Miyahara, a professor of Tokyo Medical and Dental University, and Ryouichi Sakiyama, an associate professor of Osaka Institute of Technology. The company is looking for poster themes related to research using “cell sheets” or “temperature-responsive cell cultureware.”
Establishment of a joint venture company
On January 30, 2020, a joint venture company funded by the company, MetaTech, Mr. Chen Zongji (representative director of BOÎTE À BIJOUX Investment Co., Ltd. in Taiwan), Mr. Du Yuankun (director of E-Da Hospital), and others was established. (its business operation will start in April 2020).
The joint venture company will develop products for regenerative medicine and treatment methods while applying cell sheet engineering based on the technologies provided by universities and research institutions in Japan and Taiwan by examining product overviews and optimizing manufacturing methods.
Technologies developed by Professor Du Yuankun of E-Da Hospital (Kaohsiung City, Taiwan) are one of the options. As part of the regenerative medicine supporting business, it will also provide consultancy for clinical development and support in submitting application for the manufacture and sales approval.
| Name | Up Cell Biomedical Co. |
| Location | 14-2, No.75, Section 1, Xintaiwu Road, Xizhi District, New Taipei City |
| Representative | Representative Director Wang Huijun (a guest professor of Institute of Biological Chemistry, Academia Sinica, and Acting Executive Director of National Biotechnology Research Park) |
| Business description | R&D and commercialization of the cell sheet regenerative medicine business based on the technologi Japan es provided by universities, etc. in Taiwan and. |
| Capital amount | 130,000,000 Taiwan dollars (at the time of establishment) (approx. 500 million yen) |
| Fiscal term | Fiscal term ends in December |
* Board members: Wang Huijun (Representative Director), He Hongneng (Vice-Representative Director), Setsuko Hashimoto (Director)
* Adviso Yang Zhihui Advisory Director, Scientific Advisory Board: Chen Yaochang, Huang Yanhua, Masayo Takahashi
Promotion of Business Alliance toward Global Business Expansion
The company actively participates in exhibitions inside and outside Japan for business alliance and licensing.
Results for FY 12/19
Feb. DUPHAT (Dubai) | Jun. Bio International (Philadelphia) |
Mar. Bio Asia (Tokyo) | Jul. Bio Asia Taiwan (Taipei) |
Mar. Bio Europe Spring (Vienna) | Oct. Bio Japan (Yokohama) |
May China BIO (Shanghai) | Dec. Healthcare EXPO Taiwan (Taipei) |
Schedule for FY 12/20
May China BIO (Suzhou) | Sep. BIO Partnering APAC 2020 (Shanghai) |
Jun. Bio US (San Diego) | Oct. BIOEU Fall (Germany) |
Jul. Bio Asia Taiwan (Taipei) | Dec. Healthcare EXPO Taiwan (Taipei) |
3. Trends by Segment
In the cell sheet regenerative medicine business, the company promoted research and development of mainly in-house development cell sheet regenerative medicine product pipelines for epithelial cell sheets for esophageal regeneration and the regenerated cartilage sheet, and also began to consider the development of periodontal ligament cell sheets. In the regenerative medicine supporting business, on the other hand, the company worked on research and development of new equipment and custom-made products in response to customer needs, and increased overseas sales through sales promotion activities such as participation in exhibitions, in order to expand the equipment business. In addition, the regenerative medicine consignment business, which was launched in November 2018, posted sales.
3-1 Cell sheet regenerative medicine business
Epithelial Cell Sheet for Esophageal Regeneration
22,000 patients within Japan are diagnosed with esophageal cancer every year (90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma), with 11,500 patients dying every year. In addition, the rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients, with five year survival rates for males and females said to be 36% and 44%, respectively. The endoscopic resection surgery (ESD) was posted in the drug price list from 2008 and is on the rise, but its side effect of esophageal stricture after surgery has been recognized as a problem.
The company strives to put the epithelial cell sheet for esophageal regeneration into practice, aiming to improve patients’ quality of life by reducing the frequency of occurrence of esophageal strictures.
(Source: the company)
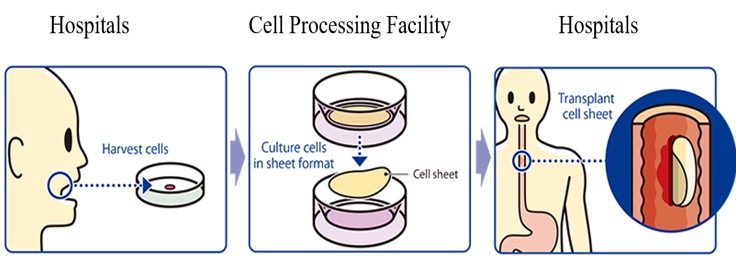
The treatment with “the epithelial cell sheet for esophageal regeneration” was developed by Tokyo Women's Medical University, in order to solve the problem with the regenerative medical treatment against esophageal cancer (treatment of esophageal tear and prevention of stenosis). Cells taken from the oral mucosa of a patient are cultured for about 2 weeks using the temperature-responsive cell cultureware to produce cell sheets. In conjunction with the process of culturing cell sheets, an endoscopic surgery for esophageal cancer excision is performed and the cell sheets are transplanted to the part of an esophageal tumor in the patient.
Efforts to obtain approval inside and outside Japan
In Japan, the company finished with case registration in the second quarter of fiscal 2018 and completed the clinical trials in the first quarter of fiscal 2019. No side effect was reported in the clinical trials, meaning that no safety issues were confirmed; on the other hand, the efficacy rate of the “effectiveness of stricture prevention 8 weeks after endoscopic submucosal dissection (ESD)” (rate of cases of non-stenosis), which was an important evaluation item, was only 12.5%, which did not prove the statistical superiority to the threshold response rate (the rate of cases of non-stenosis in patients who did not receive any treatment after ESD). Accordingly, it is necessary to conduct an additional clinical trial, and the company plans to submit a notification on the clinical trial by the end of this term.
The company is currently in discussions with the PMDA about the additional clinical trial, but the situation has changed. The Guidelines for the Treatment of Esophageal Cancer (edited by the japan Esophageal Society), which was published in 2017, strongly recommends either local steroid injection or oral steroids for prevention of stenosis after endoscopic treatment of esophageal cancer. Steroids are widely recognized as an inexpensive and effective treatment, and nowadays, steroid therapy for the prevention of stenosis seems to be more popular. This necessitated the additional clinical trial of “epithelial cell sheet for esophageal regeneration” to target patients at risk for steroid administration. Therefore, discussions will be held with the PMDA on the target patients and the number of cases required.
Outside Japan, the company signed a contract for business alliance with MetaTech in the first quarter (April.) of fiscal 2017 and MetaTech submitted a notification on a clinical trial at the end of December 2018. In Europe, it had discussions with European Medicines Agency (EMA) in fiscal 2016, to obtain approval.
Regenerated Cartilage Sheet
Regenerated cartilage sheets are the regenerative medicine products used for the treatment of slowly progressive and intractable articular cartilage degeneration. Knee osteoarthritis is refractory articular cartilage degeneration that progresses slowly. The number of its patients (aged 40 years or older) in Japan is estimated to be 25.3 million, and the number of patients suffering from symptoms is estimated to be 8 million (a survey by the 22nd Century Medical & Research Center, the University of Tokyo Hospital. According to the 2013 Comprehensive Survey of Living Conditions conducted by the Ministry of Health, Labor and Welfare, 25% of the causes for requiring support or nursing care were movement disorders).
(Source: the company)
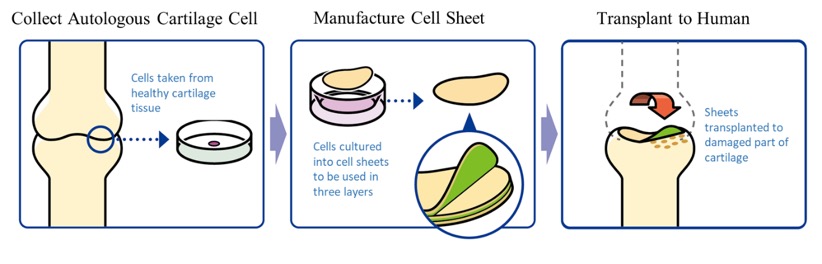
The company researched the “regenerated cartilage sheet” with Professor Masato Sato of Department of Orthopedics, Tokai University. Its indications are cartilage defects and osteoarthritis caused by sport injury and aging. As of now, there are no methods to cure the injury completely, but the collaborative research with Professor Sato is aimed at regenerating the cartilage surface radically. The cartilage of the knee is called hyaline cartilage, which is hard and excellent in cushioning and abrasion resistance properties, differing from the cartilages of the ear, nose, etc., and it is difficult to regenerate. However, it was confirmed in clinical research that the “regenerated cartilage sheet,” which is being researched collaboratively with the professor, can regenerate the cartilage of the knee as hyaline cartilage.
Prosthetic joints can be used for curing the knee osteoarthritis, but they have a service life of 15 to 20 years, so the treatment with prosthetic joints cannot be said to be radical. Compared with other companies’ regenerative medicine for knee joints, CellSeed’s treatment with cell sheets is excellent in settling into the defective cartilage, so that it can regenerate the cartilage surely.
Treatment with Autologous Cell Sheets
Professor Masato Sato started clinical research into autologous cartilage sheets in 2010 and has completed the study of 8 cases. In January 2019, “the cartilage regeneration treatment with autologous cell sheets” proposed by Tokai University Hospital was approved as Advanced Medicine B at “the 71st advanced medical care meeting” hosted by the Ministry of Health, Labor and Welfare.
In collaboration with Professor Masato Sato, the company has searched for factors that determine the efficacy of cartilage cell sheets for curing the knee osteoarthritis and conducted research for the treatment with regenerated allogeneic cartilage sheets, through the project of Japan Agency for Medical Research and Development (AMED). The company undertakes the manufacturing of the regenerated cartilage sheets, for the cartilage regeneration treatment with autologous cell sheets, which was approved as advanced medicine.
As for overseas development, the company licenses out its technology to MetaTech, and is preparing to commercialize autologous cell sheets based on the Taiwanese law (which is equivalent to Japan’s Advanced Medicine B). Also, the company, together with Professor Masato Sato, applied for and was granted a patent for “manufacturing and utilization methods of cell culture sheets” in the U.S. in November 2019. This has protected intellectual property rights in Japan, the U.S. and Europe.
Advanced Medicine B means the treatment using advanced medical technologies and the medical technologies whose efficacy and safety have satisfied certain criteria although the approval for sale is still to be obtained. Since the approval for sale has not yet been obtained, patients need to pay the treatment fee all by themselves, and treatments not covered by public health insurance cannot be combined with treatments covered by public health insurance in principle because the common parts of the treatment (medical examinations, tests, medications, hospitalization fees, etc.) are treated in the same way as public health insurance.
Treatment with allogeneic cartilage sheets
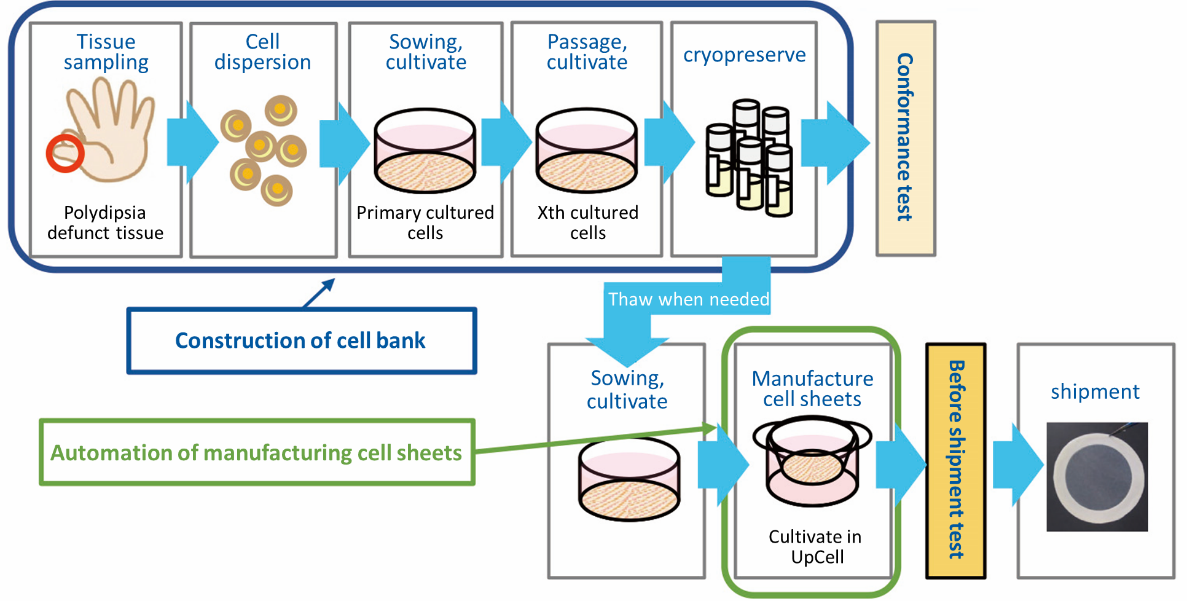
As for the treatment with allogeneic cartilage sheets, Professor Masato Sato started clinical research (transplantation of allogeneic cartilage cell sheets) in Feb. 2017, and completed transplantation of allogeneic cartilage cell sheets for 10 patients in three years (10 cases of transplantation planned in the clinical research were completed in December 2019). In parallel with clinical research, the company started to establish a cell bank and automate cell sheet production.
(Source: the company)
In the treatment with allogeneic cartilage sheets, cartilage tissue is taken from a polydactyly patient, and cultured for 2 to 3 weeks to produce cell sheets, and the cell sheets are transplanted (the cartilage cells of the finger cut from an infant who had 6 fingers congenitally, and used after obtaining consent).
However, it is necessary to develop the mechanism for providing human tissue for commercial use since it has not been developed. As the development will take time, the start of clinical trials, which was scheduled for 2021, is expected to be delayed.
The treatment with allogeneic cartilage sheets has been adopted in the project for developing evaluation methods, etc. for the industrialization of regenerative medicine (support for acceleration of development of regenerative medicine seeds) of AMED (project period: Oct. 2018 to Mar. 2021(plan)).
Regenerative Medicine Consignment Services
The company provides a wide range of regenerative medicine services on consignment in relation to cell sheet products, including development of manufacturing methods and contract manufacturing, facility management and application support, and training and education in cell culturing technology.
The company engages in manufacturing on consignment and quality testing for cell sheet products as services of development of manufacturing methods and contract manufacturing for cell sheet products. These services are characterized by a number of staff members with extensive knowledge and experience with cell culturing practices, such as clinical cultivatists certified by the Japanese Society for Regenerative Medicine, and production of cell sheets using the company’s product UpCell® at the facility with permission for manufacturing and processing specified cell products (Facility Number.: FA3160008). CellSeed received an order in November 2018 for a project related to manufacturing of the mesenchymal stem cell sheet derived from the autologous periodontal membrane used in a research project for industrializing regenerative medicine, “Periodontal Tissue Regenerative Therapy with Periodontal Ligament-Derived Mesenchymal Stem Cell Sheets,” by the Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University (with Professor Takanori Iwata as the person in charge of this research and development project), which was adopted by AMED. The order was received in November 2018, and sales were posted in the cumulative ended December 2019.
The company also offers facility management and application support services, such as preparation of application forms for manufacturing and processing specified cell products, application and submission, consulting for document preparation, maintenance of facility equipment and management systems, and management support. The training and education in cell culturing technology include training programs regarding culturing and exfoliation of cell sheets.
Acquisition of ISO9001
The company acquired ISO9001:2015, global standards for the quality management system, in order to improve reliability and customer satisfaction for cell cultureware further. The company will work to comply with laws and regulations and continuously improve its quality management system in accordance with the standards.
Applicable standard | ISO9001:2015/JIS9001:2015 |
| Date of registration | January 6, 2020 |
Registration No. | JP20/063104 |
| Expiration date | January 6, 2023 |
Scope of application | Design and production management of cell cultureware Sale of cell characteristic monitoring devices and measuring instruments |
| Certification Authority | SGS Japan Inc. |
| Certifying Body | UKAS (United Kingdom Accreditation Service) |
4. Mid-term Business Plan (FY 12/20 to FY 12/22)
4-1 Overview
The company is developing a variety of cell sheet regenerative medicine and other products based on cell sheet engineering, which is Japan’s innovative regenerative medicine technology, and aims to spread the technology around the world. In Japan, the environment surrounding regenerative medicine has changed significantly since the enactment of the Pharmaceutical and Medical Device Act and the Act on the Safety of Regenerative Medicine in November 2014, and industrialization of regenerative medicine products is progressing (in fact, seven products have been approved for manufacture and sale in the past five years).
Under these circumstances, the company has been able to expand its overseas equipment business, build a contract business structure, and enhance cooperation with MetaTech and the Taiwanese joint venture company in the regenerative medicine supporting business, etc., due to which the internal and external environments have been improved. The company intends to proceed with the plan as shown below, while taking advantage of major changes in the external environment in Japan as well as overseas.
Cell sheet regenerative medicine business
Epithelial cell sheet for esophageal regeneration | The company will take the discussions related to the additional clinical trial with the PMDA forward and aim for early application for the approval for manufacture and sales. |
Cartilage cell sheet (autologous cells) | The company will support Tokai University in working on the advanced medicine and undertake its manufacture. |
Cartilage cell sheet (allogeneic cells) | The company will accelerate the establishment of cell stock and cell sheet manufacturing automation for the early start of clinical trials. |
Third product | The company will start developing the product once the discussions with Tokyo Medical and Dental University regarding the periodontal ligament cell sheets end. |
Regenerative medicine supporting business
Entrusted manufacturing | The company will promote entrusted manufacturing and consultancy business for further revenue expansion. |
Equipment business | The company aims to promote the development of new products, secure production capacity to meet increasing demand, and expand earnings opportunities. |
Overseas development
Earnings opportunities in Taiwan | Considering the increase in investment in regenerative medicine in Taiwan, the company will strengthen cooperation with MetaTech and the joint venture company. |
Business alliance | The company will actively promote business alliance to spread Japan’s cell sheet engineering around the world. |
4-2 Numerical goals
| FY 12/ 20 Est. | FY 12/ 21 (goal) | FY 12/ 22 (goal) |
Regenerative medicine supporting business | 230 | 320 | 390 |
Cell sheet regenerative medicine business | 80 | 40 | 1,010 |
Sales | 310 | 360 | 1,400 |
Operating Income | -1,020 | -1030 | 10 |
Ordinary Income | -1,020 | -1,030 | 10 |
In the regenerative medicine supporting business, overseas sales of equipment and the regenerative medicine services are expected to be strong, while the company anticipates to generate revenue from the associated enterprises in the cell sheet generative medicine business.
5. Conclusions
The Guidelines for the Treatment of Esophageal Cancer (edited by the Japan Esophageal Society), which was published in 2017, stated, “Either local steroid injection or oral steroids is strongly recommended for prevention of stenosis after endoscopic treatment of esophageal cancer.” With this publication, steroids are becoming increasingly recognized as an inexpensive and effective treatment, and steroid treatment is adopted for the prevention of stenosis by a larger number of people. The treatment using the company’s “epithelial cell sheet for esophageal regeneration” for prevention of stenosis after endoscopic treatment of esophageal cancer is limited to only those at risk for steroid administration, which will result in fewer patients than previously expected.
But there are favorable factors. The company has delivered successful results of the regenerative medicine service from “periodontal ligament cell sheets” for Tokyo Medical and Dental University, and sales of temperature-responsive cell cultureware achieved a record high. It seems that overseas sales channels are being created. The sales of temperature-responsive cell cultureware will contribute not only to earnings, but also to increased recognition of “cell regenerative sheets,” which use fundamental technologies of “cell sheet engineering.”
Also, the company’s business in Taiwan is expanding. As it can be seen from MetaTech’s submission of a clinical trial notification for the “epithelial cell sheet for esophageal regeneration” at the end of December last year, the speed is fast. The company expects to make profit from it and the regenerated cartilage sheets at an early stage. The cartilage of the knee is called hyaline cartilage, which is hard and excellent in cushioning and abrasion resistance properties, differing from the cartilages of the ear, nose, etc., and it is difficult to regenerate. However, it was confirmed in clinical research that the “regenerated cartilage sheet,” which use fundamental technologies of “cell sheet engineering,” can regenerate the cartilage of the knee as hyaline cartilage. In addition, compared with other companies’ regenerative medicine for knee joints, CellSeed’s treatment with cell sheets is excellent in settling into the defective cartilage, so that it can regenerate the cartilage surely. Of course, expectations are high in Japan, and we look forward to the launch of advanced medicine that has already been approved and the contribution of consigned manufacturing to the company. The joint venture company established in Taiwan has been blessed with a number of partners and is expected to expand in the future.
The company’s financial base supporting business activities are sound and there is no reason to worry. We would like to pay attention to how much the company is able to grow and increase the number of positive factors in the term ending December 2020.
<Reference: Regarding Corporate Governance>
◎Organization type, and the composition of executive directors and auditors
Organization type | Company with company auditor |
Directors | 4 directors, including 2 external ones |
Auditors | 3 corporate auditors, including 2 external ones |
◎Corporate Governance Report(Latest Update:April 4, 2019)
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people’s health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.
In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)>
CellSeed has stated, “Our company implements all the basic principles stipulated in the Corporate Governance Code as a JASDAQ listed company.”
This report is intended solely for information purposes and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the company and obtained from sources that we judge to be reliable. However, we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration. Copyright(C) 2020 Investment Bridge Co., Ltd. All Rights Reserved. |
To view back numbers of Bridge Reports on CellSeed (7776) and other companies, or IR related seminars of Bridge Salon, please go to our website at the following url: www.bridge-salon.jp/


