Bridge Report:(7776)CellSeed Fiscal Year ended December 2020
 Setsuko Hashimoto, President &CEO | CellSeed Inc.(7776) |
 |
Company Information
Market | JASDAQ |
Industry | Precision Instrument (Manufacturing) |
President | Setsuko Hashimoto, Ph.D. |
HQ Address | Telecom Center Building, Aomi 2-5-10, Koto-ku, Tokyo |
Year-end | December |
Homepage |
Stock Information
Share Price | Number of shares issued (End of the Term) | Total market cap | ROE Act. | Trading Unit | |
¥256 | 16,008,319shares | ¥4,0981million | - | 100shares | |
DPS Est. | Dividend yield Est. | EPS Est. | PER Est, | BPS Act. | PBR Act. |
¥0.00 | - | ¥-62.34 | - | ¥93.85 | 2.7x |
*Stock price as of closing on March 4, 2021. Each figure is from the financial results for the Fiscal Year ended December 2020.
Earnings Trend
Fiscal Year | Sales | Operating Profit | Current Profit | Net Profit | EPS | DPS |
December 2017 | 85 | -956 | -964 | -966 | -93.29 | 0.00 |
December 2018 | 1,026 | 140 | 140 | 129 | 11.35 | 0.00 |
December 2019 | 275 | -780 | -786 | -782 | -66.60 | 0.00 |
December 2020 | 199 | -719 | -744 | -783 | -55.31 | 0.00 |
December 2021 Est. | 213 | -976 | -998 | -998 | -62.34 | 0.00 |
* The estimates were provided by the company. Units: million yen and yen.
This Bridge Report presents CellSeed Inc.’s earnings results for the Fiscal Year ended December 2020, Fiscal Year ending December 2021 earnings forecasts.
Table of Contents
Key Points
1. Company Overview
2. Fiscal Year ended December 2020 Earnings Results
3. Fiscal Year ending December 2021 Earnings Forecasts
4. Mid-Term Management Plan
5. Conclusions
<Reference: Regarding Corporate Governance>
Key Points
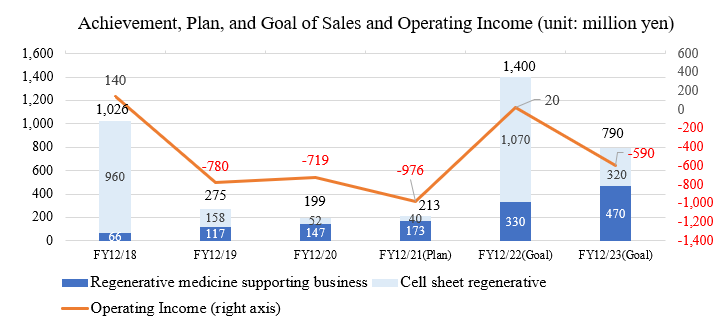
- For the term ended December 2020, sales were 199 million yen, down 27.7% year on year, and operating loss was 719million yen (780 million yen in the same period of the previous term). The sales of the regenerative medicine supporting business (mainly selling devices) increased 25.8% year on year to 147 million yen, hitting a record high successively, thanks to the good performance of overseas businesses. On the other hand, the sales of the cell sheet regenerative medicine business dropped 67.1% year on year to 52 million yen. As a result, total sales declined. As for profit and loss, the company conducted selection and concentration action for R&D theme resulting in cost reduction and decreasing operating loss.
- For the term ending December 2021, it is estimated that sales will be 213million yen, up 7.0% year on year, and operating loss will be 976 million yen (719 million yen in the previous term). The sales of the regenerative medicine supporting business is projected to be 173 million yen, hitting a record high this term, again. The company will make continuous efforts to sell mainly devices especially outside Japan. In addition, the company will proceed with the manufacturing of devices for regenerative medicine on consignment for supporting the R&D and commercialization of regenerative medicine, through comprehensive support regarding regenerative medicine. The sales of the cell sheet regenerative medicine business are forecasted to be 40 million yen. The company will continue the investment in R&D, to develop mainly epithelial cell sheets for esophageal regeneration and for cartilage regeneration. The estimated sales of 40 million yen in this segment will come from the exclusive business alliance contract with MetaTech (AP) Inc.
- The company announced its mid-term management plan for the three years from the term starting January 2021 to the term ending December 2023.
- In the cell sheet regenerative medicine business, the company will start an additional clinical trial for epithelial cell sheets for esophageal regeneration, with the aim of applying for certification for production and sale in 2025, and collect non-clinical data at a more rapid pace, to submit a plan for clinical trials for regenerated allogeneic cartilage sheets at the end of 2022. The company will redevelop collaborative businesses with MetaTech and the joint venture in Taiwan, and aim to seize more earning opportunities.
- In the regenerative medicine supporting business, the company aims to expand the overseas sales of devices by cementing the cooperation with Thermo Fisher Scientific, Inc. To do so, the company will strive to enrich and expand its manufacturing system and capability, and increase earning opportunities. In addition, the company will expand its business by developing devices and supplying them to new markets for the mass culture of cells for research, and promote the businesses of consultancy and undertaking development and manufacturing, with the aim of increasing earning opportunities.
- The company abandoned the development of a periodontal ligament cell sheet, which had been expected to become the third sheet of the company, and the development of epithelial cell sheets for esophageal regeneration in Europe, in accordance with the policy of selection and concentration, but it overcame the ethical issues in the development of cartilage cell sheets with allogeneic cells, became able to obtain cartilage cell tissue, which can be used stably for commercial purposes, and the sales of the regenerative medicine supporting business hit a record high successively. It can be said that the business foundation was fortified in the term ended December 2020.
- According to their mid-term business plan, the top line is expected to grow considerably, as the sales of the regenerative medicine supporting business will expand steadily mainly outside Japan and double year on year in the term ending December 2022, and in the cell sheet regenerative medicine business, the company will license out its technologies to some degree. There is a risk that COVID-19 will restrict the activities for new business alliances and licensing like in the previous term, but we would like to pay attention to whether or not the company will take a great leap forward.
1. Company Overview
Regenerative medicine is a new kind of medicine for regenerating and curing lost, damaged or deteriorated tissues.
CellSeed uses the fundamental technologies of “cell sheet engineering” developed in Japan by Professor Okano of the Tokyo Women’s Medical University in its “cell sheet regenerative medicine” that employs “cell sheets” for the cell regenerative medicine business, and the regenerative medicine support business, where temperature responsive cell cultureware used to fabricate cell sheets are developed and sold and the regenerative medicine consignment services, which support for research and development and commercialization of regenerative medicine, is provided.
“Cell sheet engineering” – Basic Technologies for Regenerative Medicine
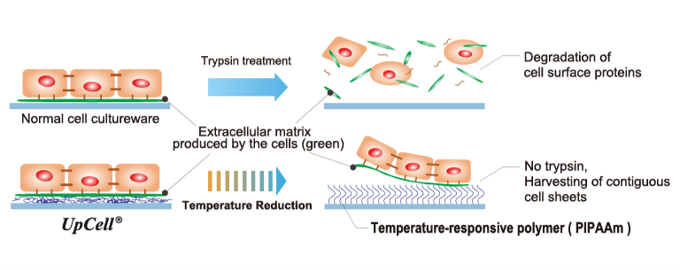 (From the company material) | “Cell sheet engineering” is the world’s first platform technology developed in Japan by Professor Emeritus Mitsuo Okano of Tokyo Women’s Medical University. Cells are cultured in cell cultureware whose surface is processed with the temperature responsive polymer that changes the molecular structure according to temperatures. The surface of the cell cultureware becomes moderately hydrophobic (waterproof) at 37 degrees Celsius to allow cells to adhere to it and hydrophilic (containing water) at a temperature of 20 degrees Celsius to prohibit cells from attaching to it. Thus, an organically combined cell sheet can be obtained from the cultureware just by changing temperatures while the extracellular matrix (adhesive proteins) is maintained. In general, cells grow by secreting the extracellular matrix and fixing themselves. In other words, they cannot develop unless they fix themselves somewhere while releasing adhesive proteins, and cells cultured are conventionally harvested by decomposing adhesive proteins using such proteolytic enzymes as trypsin (breaking down adhesive proteins was the only way to obtain cells cultured). |
Japan Leads the World in Cell Sheet Engineering.
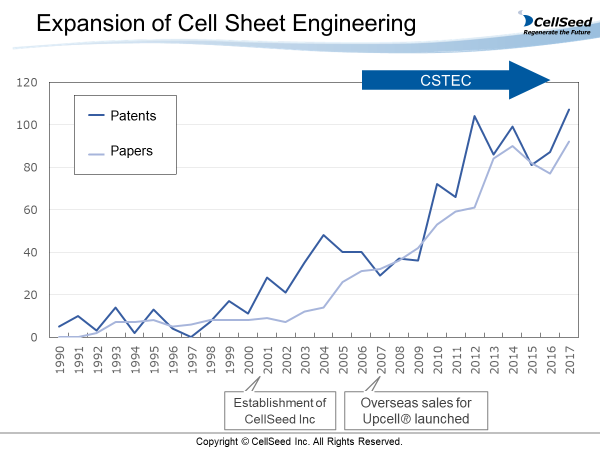
Since a paper regarding “cell sheet engineering” was published about 30 years ago, a lot of papers have been published and applications for many patents have been submitted, and in recent years, over 100 papers and patent applications have been issued every year. Namely, the research in this field has progressed in the past decade.
In addition, most of the papers and patent applications came from Japan. This is one of a few technologies in which Japan leads the world.
(From the company material)
1-1 Business model of CellSeed
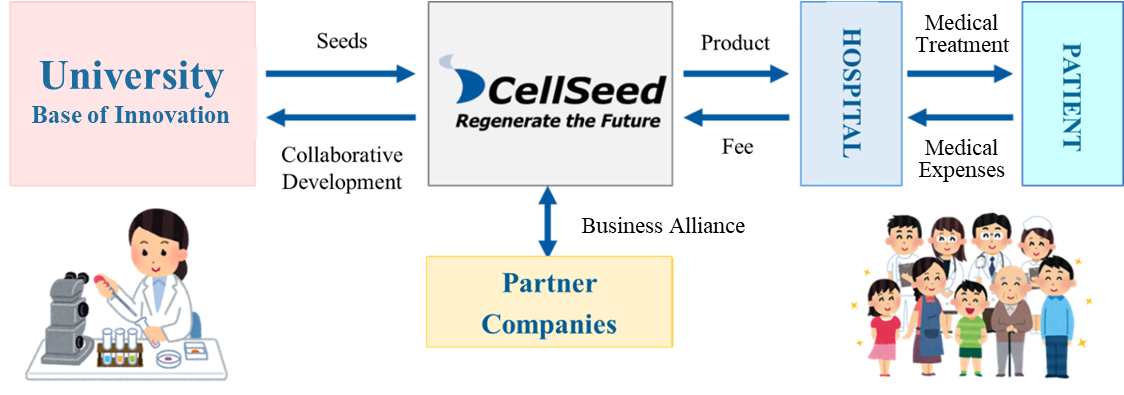
Mission: We take the initiative of contributing to global health care in the valuable and innovative field of regenerative medicine.
Using the outcomes of research into cell sheets conducted at universities as seeds, the company performs clinical trials, transforms them into regenerative medicine products, and provides products to patients.
(From the company material)
1-2 Business Description
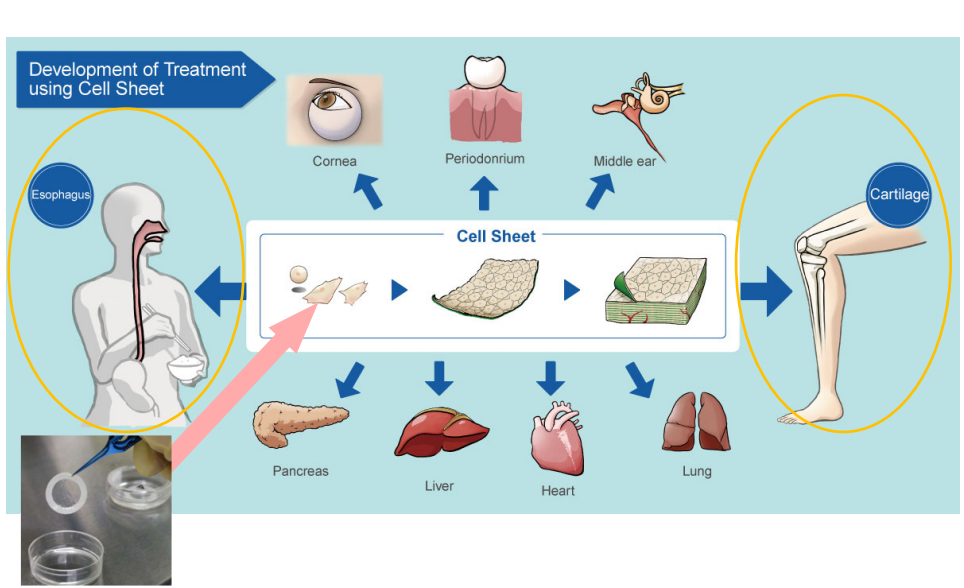
Cell sheet regenerative medicine businessThe treatment methods based on “cell sheet engineering” are targeted at various body parts, but the company currently focuses on “epithelial cell sheets for esophageal regeneration” and “cartilage cell sheets” for knee cartilage.
In April 2016, the company started clinical trials for “epithelial cell sheets for esophageal regeneration” in Japan, but failed to obtain sufficient data regarding their effectiveness. Accordingly, the company submitted a notification on additional clinical trials in October 2020. As for business in overseas nations, CellSeed entered into a business alliance with MetaTech (AP) Inc. (hereinafter referred to as MetaTech) in Taiwan in April of fiscal 2017 and the company submitted a clinical trial notification at the end of December 2018.
The “regenerated cell sheet,” for which Tokai University Hospital had submitted an application for approval, was approved as advanced medicine in January 2019, allowing the company to prepare for starting to offer the medical treatment at the university hospital. Furthermore, CellSeed has licensed out the product to MetaTech and efforts are put forth to commercialize autologous cartilage sheets in accordance with the Taiwanese law (which is equivalent to those governing Japan’s Advanced Medical Care B Program).
Development of treatment methods using “cell sheet engineering”
(From the company material)
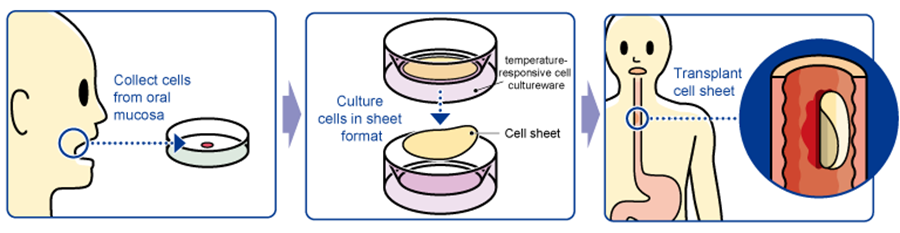
“Epithelial Cell Sheet for Esophageal Regeneration”
22,000 patients within Japan are diagnosed with esophageal cancer every year with 11,500 patients dying every year. The rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients. In addition, 90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma and five years survival rates for males and females, which is said to be 41% and 46%, respectively, are under 50%. he endoscopic resection surgery (ESD) was posted in the drug price list from 2008 and is on the rise, but its side effect of esophageal stricture after surgery has been recognized as a problem.
However, introduction of epithelial cell sheets for esophageal regeneration will allow esophageal strictures to develop less frequently, which is expected to improve the quality of life of patients.
The treatment with “the epithelial cell sheet for esophageal regeneration” was developed by Tokyo Women's Medical University, in order to solve the problem with the regenerative medical treatment against esophageal cancer (treatment of esophageal tear and prevention of stenosis). Cells taken from the oral mucosa of a patient are cultured for about 2 weeks using the temperature-responsive cell cultureware to produce cell sheets. In conjunction with the process of culturing cell sheets, an endoscopic surgery for esophageal cancer excision is performed and the cell sheets are transplanted to the part of an esophageal tumor in the patient. Clinical studies were conducted at universities between 2008 and 2014: 10 cases at Tokyo Women's Medical University, 10 cases at Tokyo Women's Medical University and Nagasaki University (long-distance transport validation: cells collected at Nagasaki University were cultured at Tokyo Women's Medical University and transplanted at Nagasaki University), 10 cases at Karolinska University Hospital (Sweden), for a total of 30. The company signed a basic development agreement with Tokyo Women's Medical University and took over the university's research results for commercialization.
The company submitted a plan for clinical trials in April 2016 and finished the trials in March 2019, but failed to prove statistical superiority, and additional clinical trials became necessary. After consulting with PMDA about additional clinical trials, the company submitted a plan for additional clinical trials in October 2020, and the first case was registered in February 2021.
Outside Japan, the company licensed MetaTech in Taiwan, with which it formed an alliance in April 2017, to use the sheet. In 2018, MetaTech submitted a plan for clinical trials.
Meanwhile, the company was proceeding with development in Europe, based on a subsidiary in Sweden, but it decided to stop the development in 2020, because endoscopic therapy had not been diffused in Europe as expected and the company had to concentrate on the acquisition of approval for production and sale in Japan.
(From the company material)
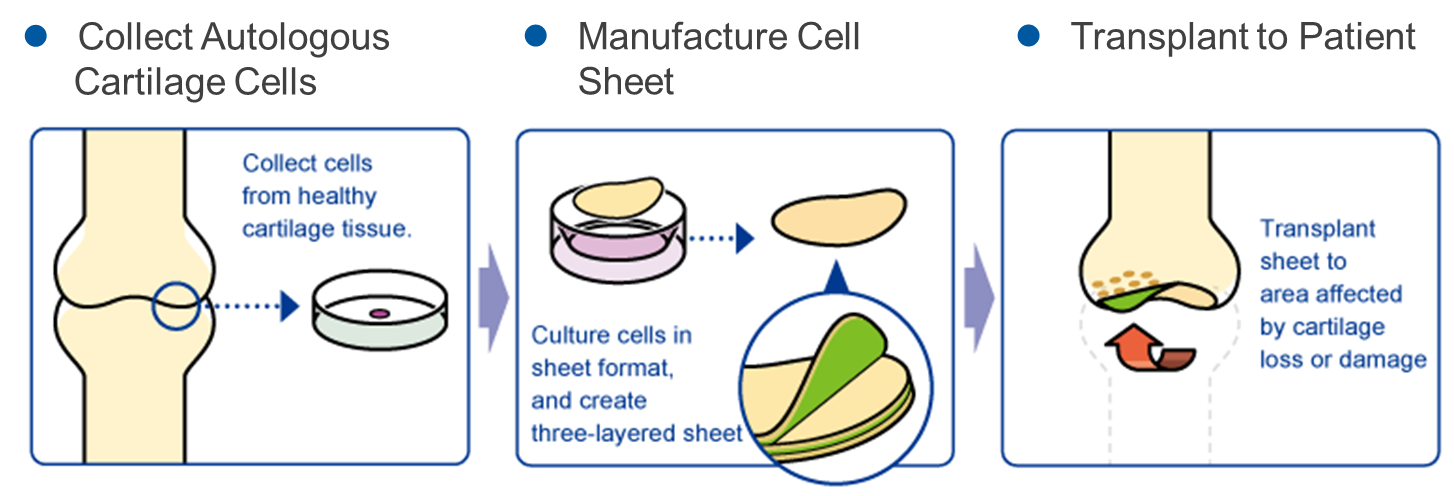
“Regenerated Cell Sheet”
The company researched the “regenerated cell sheet” with Professor Masato Sato of Department of Orthopedics, Tokai University. Its indications are cartilage defects and osteoarthritis caused by sport injury and aging.
Knee osteoarthritis is refractory articular cartilage degeneration that progresses slowly. The number of potential patients in Japan is estimated to be about 30 million, of which about 10 million patients are thought to have subjective symptoms. Furthermore, population aging in Japan is expected to raise the number of patients diagnosed with the illness, making it a disease that needs to be dealt with immediately from the perspective of citizens’ healthy life expectancy and costs of long-term care and medical services. As of now, there are no methods to cure the injury completely, but the collaborative research with Professor Masato Sato is aimed at regenerating the cartilage surface radically. The cartilage of the knee is called hyaline cartilage, which is hard and excellent in cushioning and abrasion resistance properties, differing from the cartilages of the ear, nose, etc., and it is difficult to regenerate. However, it was confirmed in clinical research that the “regenerated cartilage sheet,” which is being researched collaboratively with the professor, can regenerate the cartilage of the knee as hyaline cartilage.
Professor Masato Sato started clinical research into autologous cartilage sheets in 2010 and has completed the study of 8 cases. In January 2019, “the cartilage regeneration treatment with autologous cell sheets” proposed by Tokai University Hospital was approved as Advanced Medicine B at “the 71st advanced medical care meeting” hosted by the Ministry of Health, Labor and Welfare.
CellSeed will manufacture regenerative cartilage sheets on consignment for treating patients by regenerating cartilage using autologous cell sheets as an advanced medical care program, and a surgery of the first patient under the category of Advanced Medical Care B Program was completed in August 2020.
The licensee MetaTech in Taiwan started the commercialization of autologous cartilage sheets in accordance with the Taiwanese law (complying with Advanced Medicine B in Japan), and performed transplantation for 10 patients. Then, CellSeed received a milestone revenue of 50 million yen.
In addition, in November of 2019, CellSeed and Tokai University jointly applied for a patent of “Cell Culture Sheet and Manufacturing Method and Use Thereof,” one of the outcomes of the joint research with Professor Masato Sato of the university, in the U.S. and the patent application was approved. This ensures that the intellectual property right of the product is now protected in Japan, the United States of America, and Europe.
(Source: the company)
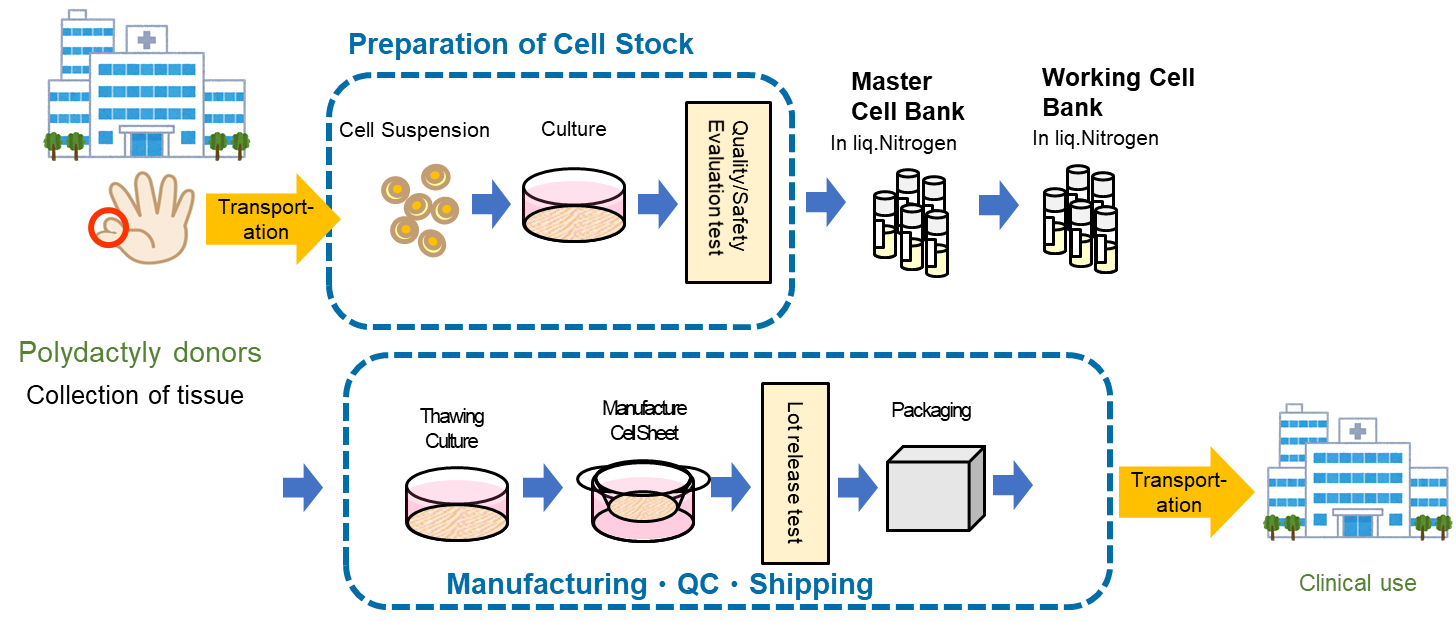
The company is also proceeding with the R&D of treatments with allogeneic cartilage sheets, which are derived from cells of people other than patients.
The professor Masato Sato started clinical research regarding the transplantation of allogeneic cartilage cell sheets in February 2017, and performed transplantation for 10 patients in 3 years. In December 2019, the 10th transplantation was finished.
In parallel with clinical research, a cell bank will be established and manufacturing of cell sheets will be automated. The treatment with allogeneic cartilage sheets has been adopted in the project for developing evaluation methods, etc. for the industrialization of regenerative medicine (support for acceleration of development of regenerative medicine seeds) of AMED (project period: Oct. 2018 to Mar. 2021(plan)).
For developing cartilage cell sheets with allogeneic cells, the discarded tissue of patients with polydactyly who have six fingers, so it is necessary to solve ethical issues, but in December 2020, the company obtained approval for the provision of cartilage tissue collected from patients with polydactyly from National Center for Child Health and Development. Accordingly, the company is now able to obtain the tissue of cartilage cells in a stable manner. This will accelerate R&D for receiving approval for clinical trials, manufacturing, and sale.
At the symposium of the Japanese Society for Regenerative Medicine held in March 2021, the company gave a poster presentation.
<Allogeneic cells> Development of cartilage cell sheets
The company is also implementing measures for expanding its business in new fields.
In November 2020, the company signed a contract for technological transfer with KanonCure Inc., which is a venture company from Tottori University, for the manufacturing of products for clinical trials of cell sheets for treatment of liver disease using mesenchymal stem cells, which are being developed by KanonCure, as products for regenerative medicine, etc.
It is said that about 53,000 people suffer from liver disease and about 2,200 patients need liver transplantation every year, but the number of patients who can undergo liver plantation is only about 500 per year. Based on the accumulated know-how for commissioned manufacturing of cell sheets and the technological information from KanonCure, CellSeed will discuss necessary technologies for manufacturing hepatic cell sheets and related technologies, transfer manufacturing methods, and prepare for concluding a contract with KanonCure for entrustment of manufacturing of products for clinical trials.
Regenerative Medicine Consignment Services
The company provides regenerative medicine services on consignment in relation to temperature responsive cell cultureware, etc., including development, manufacture, and sales and cell sheet products, including development of manufacturing methods and contract manufacturing, facility management and application support, and training and education in cell culturing technology.
In the commissioned manufacturing of cell sheet products, the company develops and manufactures mainly cell sheets on consignment for pharmaceutical companies and research institutions. The company has a number of staff members with extensive knowledge and experience with cell culturing practices, such as clinical cultivatists certified by the Japanese Society for Regenerative Medicine, and they do those services at the facility with permission for manufacturing and processing specified cell products. The cartilage regeneration sheet was approved in January 2019 for the advanced medical treatment B that Tokai University had applied for, and began to be cultured by the company at its cell culture center (contract manufacturing) in 2020.
Cell Culture Center
The cell sheets used for advanced medicine are cultivated at the cell culture center of CellSeed on commission.
With a floor space of about 763 square meters, the Cell Culture Center is equipped with an automated monitoring system that controls the cleanliness, room pressure, temperature and humidity, and operational status of equipment (such as incubators and reagent stockers), and a surveillance camera system throughout the entire facility. Besides, the facility is only twenty-minute drive from Haneda International Airport, making it possible and easy to transport products by air. In March 2017, a license to manufacture and process specified cell products as per the provisions set forth in Paragraph 1, Article 35 of the Act on Safety of Regenerative Medicine was granted by the Ministry of Health, Labour and Welfare. Consequently, CellSeed is able to process specified cell products on consignment.

(From the company material)
2. Fiscal Year ended December 2020 Earnings Results
2-1 Consolidated Earnings
| FY 12/ 19 | Share | FY 12/ 20 | Share | YoY Change |
Sales | 275 | 100.0% | 199 | 100.0% | -27.7% |
Gross Income | 216 | 78.5% | 138 | 69.3% | -36.2% |
SG&A | 997 | 361.6% | 857 | 430.0% | -14.0% |
R&D | 546 | 198.0% | 438 | 220.0% | -19.7% |
Operating Income | -780 | - | -719 | - | - |
Ordinary Income | -786 | - | -744 | - | - |
Net Income | -782 | - | -783 | - | - |
* unit: million yen
Sales were 199 million yen, down 27.7% year on year, and operating loss was 719 million yen (780 million yen in the same period a year ago).
The sales of the regenerative medicine supporting business (mainly Cell cultureware ) increased 25.8% year on year to 147 million yen, hitting a record high successively, thanks to the good performance of overseas businesses. On the other hand, the sales of the cell sheet regenerative medicine business dropped 67.1% year on year to 52 million yen. As a result, total sales declined. As for profit and loss, the company conducted selection and concentration measures, reducing costs for R&D, etc. and decreasing operating loss.
| FY 12/ 19 | Share | FY 12/ 20 | Share | YY Change |
Regenerative medicine supporting business | 117 | 42.5% | 147 | 73.9% | 25.8% |
Cell sheet regenerative medicine business | 158 | 57.5% | 52 | 26.1% | -67.1% |
Sales, Total | 275 | 100.0% | 199 | 100.0% | -27.7% |
Regenerative medicine supporting business | -43 | - | -38 | - | - |
Cell sheet regenerative medicine business | -424 | - | -390 | - | - |
Adjustments | -312 | - | -290 | - | - |
Operating Income, Total | -780 | - | -719 | - | - |
* Unit: million yen
Regenerative Medicine Supporting Business
Sales stood at 147 million yen, up 25.8% from year on year, and operating loss was 38 million yen (an operating loss of 43 million yen was recorded in the same period a year ago).
The company started offering products to new markets for mass culture of cells for research, which will be used for developing methods for preventing and curing a variety of infectious diseases, such as COVID-19, and cancers. It cemented the cooperation with existing distributors for increasing the sales of devices and continued active sales promotion.
As a result, mainly overseas sales grew steadily, and sales hit a record high successively.
In the commissioned regenerative medicine business, in which the company supports regenerative medicine by utilizing the cell culture center, the treatment of Advanced Medicine B with autologous cartilage regeneration sheets was started in Tokai University, which is a partner for collaborative research. The company is entrusted by Tokai University with manufacturing, and some surgical operations were put off due to the spread of COVID-19, so sales fell below the initial estimate, but the company posted sales from 3 cases.
In November 2020, the company signed a contract for technological transfer with KanonCure Inc., which is a venture company from Tottori University, for the manufacturing of products for clinical trials of cell sheets for treatment of liver disease using mesenchymal stem cells, which are being developed by KanonCure, as products for regenerative medicine, etc.
Cell Sheet Regenerative Medicine Business
Sales were 52 million yen, down 67.1% year on year, and operating loss stood at 390 million yen (loss of 424 million yen in the same period of the previous year).
In November 2020, the company posted sales of 50 million yen from MetaTech. The company was supposed to receive a milestone revenue of 50 million yen (a remuneration for achieving a goal) at the time of completion of treatments with cartilage regeneration sheets for 10 patients, based on the contract for licensing MetaTech to exclusively develop, manufacture, and sell epithelial cell sheets for esophageal regeneration and cartilage regeneration sheets in Taiwan (concluded in April 2017) in the cell sheet regenerative medicine business.
2-2 Financial Condition and Cash Flows (CF)
Summary of BS
| December 19 | December 20 |
| December 19 | December 20 |
Cash | 1,065 | 1,460 | Accounts payable | 33 | 41 |
Receivables | 56 | 45 | Income taxes payable | 10 | 18 |
Inventories | 48 | 42 | Advances received | 30 | 28 |
Prepaid expenses | 19 | 20 | Accounts payable and others | 36 | 31 |
Current Assets | 1,245 | 1,622 | Interest-bearing debt | - | 160 |
Tangible Assets | 29 | - | Liabilities | 110 | 280 |
Investment & Others | 181 | 184 | Net Assets | 1,345 | 1,526 |
Fixed assets | 210 | 184 | Total Liabilities and Net Assets | 1,456 | 1,806 |
* Unit: million yen
Total assets stood at 1,806 million yen, up 395 million yen from the end of the previous term. Cash and deposits increased, due to the exercise of share acquisition rights. As for liabilities and net assets, capital and capital surplus increased due to the issuance of new shares through the exercise of share acquisition rights, but a net loss was posted, so the deficit of retained earnings augmented.
CF
| FY12/19 | FY12/20 | YoY Change |
Operating Cash Flow | -577 | -700 | -123 |
Investing Cash Flow | -133 | -12 | +120 |
Free Cash Flow | -710 | -713 | -2 |
Financing Cash Flow | 721 | 1,102 | +381 |
Cash and Equivalents at the end of term | 1,065 | 1,460 | +395 |
* Unit: million yen
The revenues from the issuance of shares through the exercise of share acquisition rights increased, and the surplus of financing CF grew.
The cash position improved.
3. Fiscal Year ending December 2021 Earnings Forecasts
3-1 Consolidated earnings forecasts
| FY 12/ 20 Act. | Share | FY 12/ 21 Est. | Share | YoY Change | |
Sales | 199 | 100.0% | 213 | 100.0% | +13 | |
Operating Income | -719 | - | -976 | - | -256 | |
Ordinary Income | -744 | - | -998 | - | -253 | |
Net Income | -783 | - | -998 | - | -214 | |
* unit: million yen
Sales will be 213 million yen, up 7.0% year on year, and operating loss will be 976 million yen (719 million yen in the previous term).
The sales of the regenerative medicine supporting business is projected to be 173 million yen, hitting a record high this term, again . The company will make continuous efforts to sell mainly devices especially outside Japan. In addition, the company will proceed with the manufacturing of devices for regenerative medicine on consignment for supporting the R&D and commercialization of regenerative medicine, through comprehensive support regarding regenerative medicine.
The sales of the cell sheet regenerative medicine business are forecasted to be 40 million yen. The company will continue the investment in R&D, to develop mainly epithelial cell sheets for esophageal regeneration and for cartilage regeneration. The estimated sales of 40 million yen in this segment will come from the exclusive business alliance contract with MetaTech (AP) Inc.
4. Mid-term management plan
The company announced the mid-term management plan for the 3 years from the term ending December 2021 to the term ending December 2023.
(1) Activities in each business
Business | Outline |
Cell sheet regenerative medicine business | *To start additional clinical trials for epithelial cell sheets for esophageal regeneration, and aim to apply for certification for manufacturing and sale in 2025. *To accelerate the acquisition of non-clinical data for submitting a plan for clinical trials for allogeneic cartilage regeneration sheets at the end of 2022 *To redevelop collaborative business with MetaTech and a joint venture in Taiwan, with the aim of increasing earning opportunities *To actively form business alliances for diffusing cell sheet engineering created in Japan, with the aim of increasing revenues |
Regenerative medicine supporting business | *To cement the cooperation with Thermo Fisher Scientific, Inc. with the aim of increasing overseas sales of devices *To expand business by developing and supplying devices for new markets for mass culture of cells for research *To enrich and expand production systems and capabilities to meet overseas increasing sales and supply of devices to new markets, and aim to increase earning opportunities *To proceed with the businesses of development, commissioned manufacturing, and consulting, with the aim of increasing earning opportunities |
(Major points)
*Epithelial cell sheets for esophageal regeneration
The company plans to apply for the certification for manufacturing and sale in 2025. It will have discussions for shortening the period of each clinical trial by increasing facilities for clinical trials, etc.
*Cartilage regeneration sheets
In December 2020, the company obtained the approval of the ethical review committee of National Center for Child Health and Development for the provision of cartilage tissue collected from patients with polydactyly, and became able to stably get cartilage tissue that can be used for commercial purposes. The company will accelerate R&D for obtaining approval for clinical trials, manufacturing, and sale of allogeneic cartilage cell sheets, and plans to submit a plan for clinical trials by the end of 2022.
*Business tie-up
In addition to the milestone income in the previous term, the company is scheduled to receive several-percent royalties according to the sales of autologous cartilage regeneration sheets from MetaTech. The company will make efforts to form new business alliances and find licensees in Asian countries, especially China, but any contracts were not signed in 2020, due to the differences in regulations, etc. The company will keep striving to form business alliances and find licensees with the aim of expanding the cell sheet regenerative medicine business inside and outside Japan while improving its business value by developing pipelines.
*Regenerative medicine supporting business
The company will enhance the development of new devices considering customer needs and emerging demand. For sales, the company will collect and analyze information on sales, etc. provided by major Japanese distributors and conduct marketing cooperatively, for the purpose of promoting the sales of devices, mainly temperature-responsive cell culture devices, and strengthen cooperation for boosting not only domestic sales, but also overseas sales of mainly Thermo Fisher.
The company will strive to enrich and expand its production systems for supplying products to new markets for mass culture of cells for research and expanding overseas sales, while maintaining the stable supply of products.
*Commissioned regenerative medicine business
The company will operate the consulting business by utilizing a variety of know-how acquired through the manufacturing of cell sheets entrusted by universities, enterprises, etc., commissioned development, and the cell sheet regenerative medicine business, with the aim of increasing earning opportunities.
*Establishment of a joint venture in Taiwan
Based on the new technologies provided by Japanese or Taiwanese universities and research institutes, the company will conduct R&D of products and methods for regenerative medicine by applying cell sheet engineering, have discussions on indications and optimize manufacturing methods for commercialization. A technology developed by Professor Du Yuan Kun of E-Da Hospital is one of candidates. In the regenerative medicine supporting business, the company plans to offer consulting services for R&D and support the application for approval for manufacturing and sale, and earn sales by receiving fees for technical instructions, etc.
*Epithelial cell sheets for cornea regeneration
The company will continue discussions with related institutions.
(2) Numerical goals
In the regenerative medicine supporting business, the company will strive to expand overseas sales and grow its business steadily.
In the cell sheet regenerative medicine business, the company will seek overseas licensees.
5. Conclusions
The company abandoned the development of a periodontal ligament cell sheet, which had been expected to become the third sheet of the company, and the development of epithelial cell sheets for esophageal regeneration in Europe, in accordance with the policy of selection and concentration, but it overcame the ethical issues in the development of cartilage cell sheets with allogeneic cells, became able to obtain cartilage cell tissue, which can be used stably for commercial purposes, and the sales of the regenerative medicine supporting business hit a record high successively. It can be said that the business foundation was fortified in the term ended December 2020.
According to their mid-term management plan, the top line is expected to grow considerably, as the sales of the regenerative medicine supporting business especially cell cultureware (devise) will expand steadily mainly outside Japan and double year on year in the term ending December 2022, and in the cell sheet regenerative medicine business, the company will license out its technologies to some degree. There is a risk that COVID-19 will restrict the activities for new business alliances and licensing like in the previous term, but we would like to pay attention to whether or not the company will take a great leap forward.
<Reference: Regarding Corporate Governance>
◎Organization type, and the composition of executive directors and auditors
Organization type | Company with audit and supervisory committee |
Directors (excluding audit and supervisory committee members) | 3 directors, including 1 external ones |
Auditors and supervisory committee members | 3 committee members, corporate auditors, including 3 external ones |
*At the annual meeting of shareholders held on March 26, 2021, the company shifted to a company with audit and supervisory committee as mentioned above.
◎Corporate Governance Report(Latest Update:March 29, 2021)
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people’s health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.
In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)>
CellSeed has stated, “Our company implements all the basic principles stipulated in the Corporate Governance Code as a JASDAQ listed company.”
This report is intended solely for information purposes and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the company and obtained from sources that we judge to be reliable. However, we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration. Copyright(C) Investment Bridge Co., Ltd. All Rights Reserved. |
To view back numbers of Bridge Reports on CellSeed (7776) and other companies, or IR related seminars of Bridge Salon, please go to our website at the following url: www.bridge-salon.jp/


