Bridge Report:(7776)CellSeed the second quarter of the Fiscal Year ending December 2022
 Setsuko Hashimoto, President | CellSeed Inc.(7776) |
 |
Company Information
Market | TSE Growth Market |
Industry | Precision Instrument (Manufacturing) |
President | Setsuko Hashimoto |
HQ Address | Telecom Center Building, Aomi 2-5-10, Koto-ku, Tokyo |
Year-end | December |
Homepage |
Stock Information
Share Price | Number of shares issued (End of the Term) | Total market cap | ROE Act. | Trading Unit | |
¥158 | 22,159,419shares | ¥3,501million | - | 100shares | |
DPS Est. | Dividend yield Est. | EPS Est. | PER Est, | BPS Act. | PBR Act. |
¥0.00 | - | ¥-43.49 | - | ¥56.44 | 2.8x |
*Stock price as of closing on September 1, 2022. The values of Share Outstanding, DPS, EPS were taken from the brief report on financial results for the second quarter of the term ending December 2022. ROE, BPS are actual results for the previous term.
Consolidated Earnings Trend
Fiscal Year | Sales | Operating Profit | Current Profit | Net Profit | EPS | DPS |
December 2018 | 1,026 | 140 | 140 | 129 | 11.35 | 0.00 |
December 2019 | 275 | -780 | -786 | -782 | -66.60 | 0.00 |
December 2020 | 199 | -719 | -744 | -783 | -55.31 | 0.00 |
December 2021 | 161 | -864 | -887 | -914 | -53.18 | 0.00 |
December 2022 Est. | 209 | -834 | -834 | -838 | -47.19 | 0.00 |
* The estimates were provided by the company. Units: million yen and yen. Net profit is the profit attributable to owners of parent. Consolidated until FY12/09; non-consolidated in FY12/10.
This Bridge Report presents CellSeed Inc.’s earnings results for the second quarter of the Fiscal Year ending December 2022 and other financial details.
Table of Contents
Key Points
1. Company Overview
2. Second quarter of the Fiscal Year ending December 2022 Earnings Results
3. Fiscal Year ending December 2022 Earnings Forecasts
4. Conclusions
<Reference1: Mid-term Management Plan>
<Reference2: Regarding Corporate Governance>
Key Points
- In the second quarter of the term ending December 2022, sales decreased, and loss shrank. Sales were 74 million yen, down 8.6% year on year. An operating loss of 348 million yen was posted. A loss of 466 million yen was recorded in the same period of the previous year. Both sales and profit were almost in line with the forecast. (The figures were consolidated in the same period of the previous year and non-consolidated in the current quarter. Increases and decreases are hereinafter reference values calculated by Investment Bridge).
- The regenerative medicine supporting business posted sales of 70 million yen and an operating loss of 40 million yen (a loss of 19 million yen was recorded in the same period last year). The company has newly established a development and manufacturing facility for cell cultureware and started full-scale operation in September 2021. With the full-scale operation of this facility, the company will not only sell cell cultureware in the regenerative medicine market, but also sell cell cultureware products for new applications for culturing substantial amounts of research cells and mainly respond to solid overseas demand. In April 2022, the company also started a support service for users of cell cultureware. Regarding the cell sheet regenerative medicine business, which supports regenerative medicine using the cell culture center, the company was entrusted with manufacturing autologous cartilage cell sheets for advanced medical treatment by Tokai University, a joint research partner, and recorded sales for one case.
- The cell sheet regenerative medicine business sales were 4 million yen, and the operating loss was 207 million yen (a loss of 319 million yen in the same period last year). Additional clinical trials are ongoing for the epithelial sheet pipeline for esophageal regeneration, and the application for manufacturing and marketing approval is scheduled for 2025. Although overseas expansion has been delayed due to the spread of the novel coronavirus, the company will continue to support MetaTech's epithelial cell sheets for esophageal regeneration and cartilage cell sheet businesses. The company will also actively negotiate with potential business partners other than Taiwan.
- The forecast remains unchanged. Sales for the term ending December 2022 are expected to increase 47 million yen from the previous term to 209 million yen. Ordinary loss is forecast to decrease 53 million yen to 834 million yen. In the regenerative medicine supporting business, the company will continue to expand cell cultureware equipment sales, especially overseas. The new product UpCell® flasks and inserts are scheduled to go on sale next year. In addition, through comprehensive support for regenerative medicine, the company will promote the commissioned manufacturing of regenerative medicine products that supports research, development, and commercialization of regenerative medicine. The commissioned manufacturing of autologous chondrocyte sheets is scheduled to record sales for multiple cases from the third quarter onward. Based on these, segment sales are expected to be 209 million yen.
- The company will continue to develop mainly epithelial cell sheets for esophageal regenerations and allogeneic chondrocyte sheets in the cell sheet regenerative medicine business. In addition, the company will continue to actively negotiate with potential business partners outside of Asia in Europe and the United States to license out pipeline technology. In April 2022, a patent was granted and registered in Japan for a "tissue regenerative cultured cell sheet, manufacturing method, and how to use it."
- The first half of the term ending December 2022 witnessed progress in the development of cell engineering sheets, reaching an agreement with the National Center for Child Health and Development on the basic terms of a contract to provide resected tissue during polydactyly (toe) surgery for industrial use and launching joint research on new treatments for central nerve injury-related diseases. We will continue to pay attention to the earnings generated by the new product, UpCell® flasks and inserts, schedule next year, and to whether the submission of the clinical trial notification for the allogeneic chondrocyte sheet, which is expected by the end of this year, would progress as planned.
1. Company Overview
[1-1 Regenerative medicine of CellSeed]
Regenerative medicine is a new kind of medicine for regenerating and curing lost, damaged or deteriorated tissues.
CellSeed uses the fundamental technologies of “cell sheet engineering” developed in Japan by Professor Okano of the Tokyo Women’s Medical University in its “cell sheet regenerative medicine” that employs “cell sheets” for the cell regenerative medicine business, and the regenerative medicine support business, where temperature responsive cell cultureware used to fabricate cell sheets are developed and sold and the regenerative medicine consignment services, which support for research and development and commercialization of regenerative medicine, is provided.
“Cell sheet engineering” – Basic Technologies for Regenerative Medicine
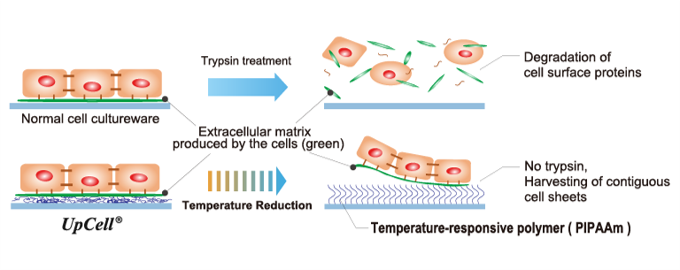
(From the company material)
"Cell sheet engineering" is a platform technology originating in Japan and the first of its kind in the world, which was invented by Mr. Teruo Okano, a professor emeritus of Tokyo Women's Medical University. Cells are cultured in “UpCell®,” a cell culture dish whose surface is processed with a temperature-responsive polymer that changes its molecular structure with temperature. The surface of a cell culture dish becomes moderately hydrophobic (water-repellent) at 37 °C, at which cells can attach, and hydrophilic (water-absorbent) at 20 °C, at which cells cannot attach. Therefore, by simply changing temperature, the organically bound "cell sheet" which retains the extracellular matrix (adhesion protein) can be recovered from the culture dish.
In general, cells secrete an extracellular matrix and grow by fixing themselves. In other words, cells cannot grow unless they are fixed somewhere while secreting adhesion proteins. However, in the conventional culture method, adhesion proteins are decomposed and recovered from cultured cells using proteolytic enzymes such as trypsin (there was no method for recovering cultured cells other than by decomposing adhesion proteins).
Huge Regenerative Medicine Market
The market size of regenerative medicine is expected to reach 2.5 trillion yen in Japan and 38 trillion yen worldwide in 2050, and a significant economic effect is expected.
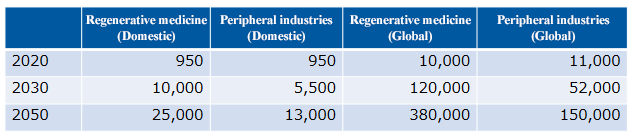
(From the company material, Unit: hundred million yen)
Expanding cell cultureware market
In recent years, along with progress in regenerative medicine research, there have been active efforts to manufacture biopharmaceuticals using substantial amounts of cultured cells, develop immunotherapy using cells, and solve food and environmental problems.
Currently, when using proteolytic enzymes, which is a commonly used cell collection technique, cells are collected in a damaged state, and it is difficult to completely maintain the original functions and components of cells. Introducing the company's temperature-responsive cell cultureware products makes it possible to collect cells without damage.
As a result, it is possible to use cells while maintaining all the functions and components that cells originally had. Thus, the company's products are attracting growing attention for their potential to significantly improve industrial efficiency and effectiveness in new markets.
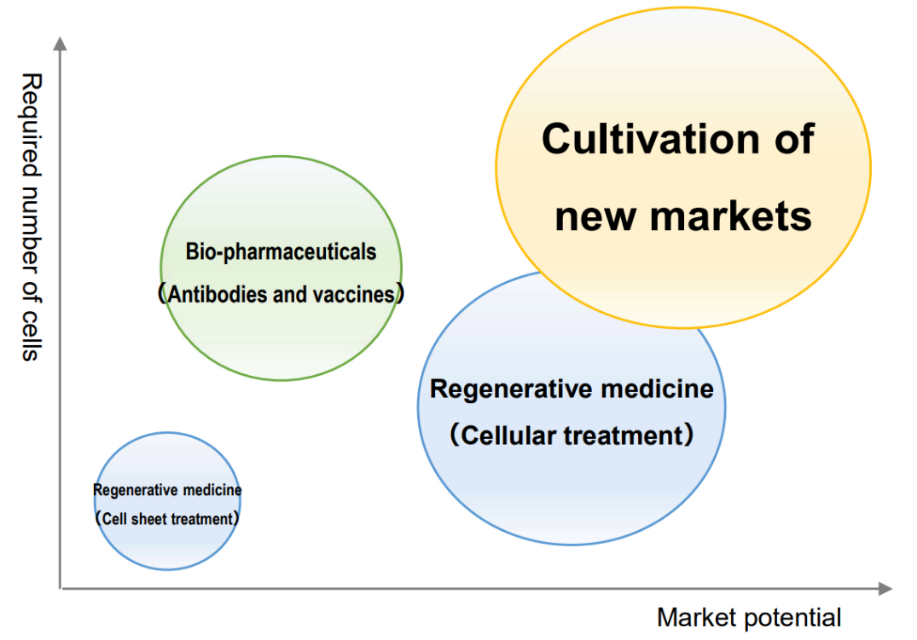
(From the company material)
Japan Leads the World in Cell Sheet Engineering.
A paper on "cell sheet engineering" was published about 30 years ago, and since then many papers have been published and patent applications have been filed. It is one of the few technologies in which Japan has taken the lead in patent applications and research papers in the life science field. Since the global launch of “UpCell®,” both patent applications and published papers have been increasing.
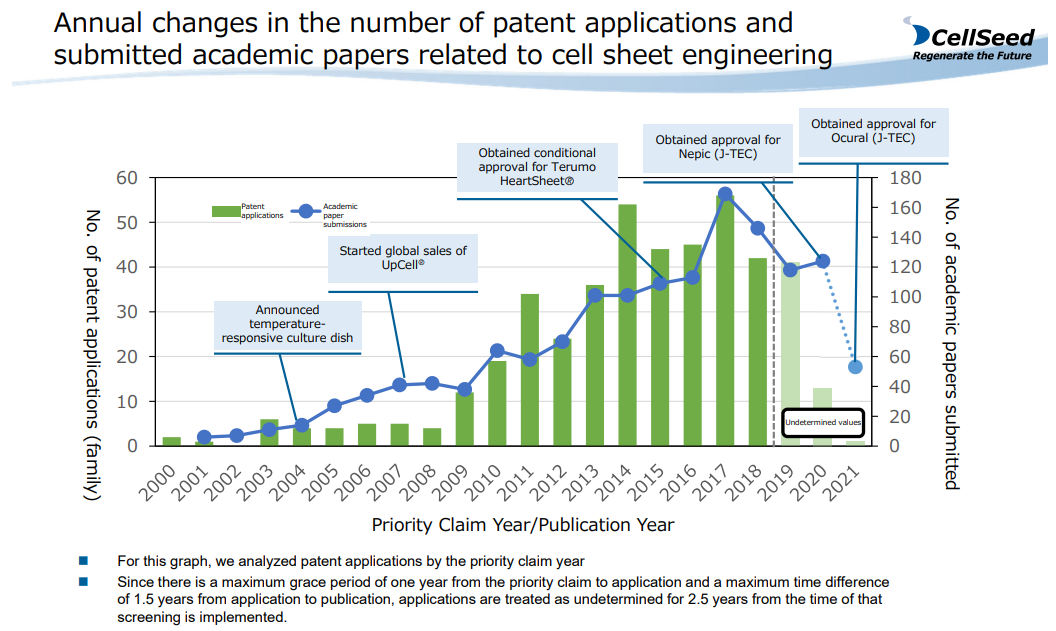
(From the company material)
[1-2 Business model of CellSeed]
Mission: We take the initiative of contributing to global health care in the valuable and innovative field of regenerative medicine.
Using the outcomes of research into cell sheets conducted at universities as seeds, the company performs clinical trials, transforms them into regenerative medicine products, and provides products to patients.
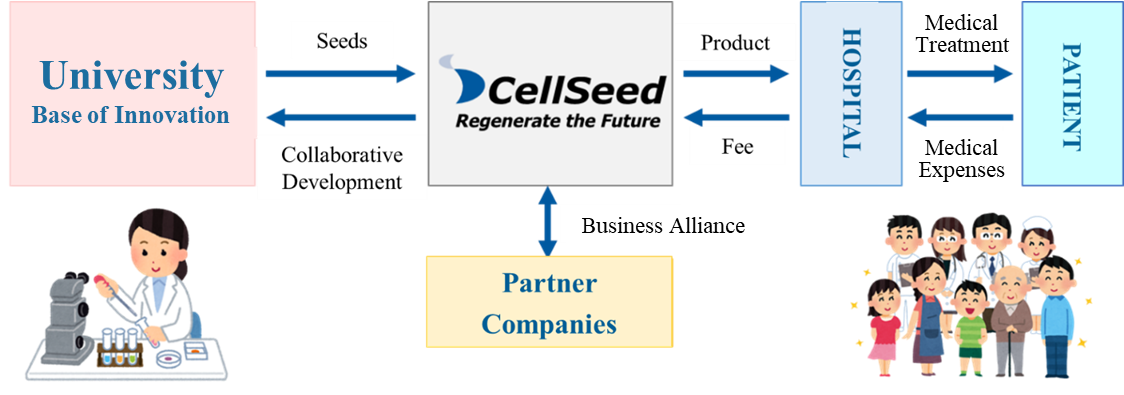
(From the company material)
[1-3 Business Description]
(1) Cell sheet regenerative medicine business
The development of treatments based on cell sheet engineering is used for various body parts. The company has also started joint research with Hokkaido University on new treatments for central nerve injury-related diseases while focusing on two products: the epithelial sheet for esophageal regeneration and the cartilage cell sheet for the knee cartilage.
In April 2016, the company started clinical trials for “epithelial cell sheets for esophageal regeneration” in Japan, but failed to obtain sufficient data regarding their effectiveness. For this reason, an additional clinical trial notification was submitted in October 2020, and the first case was registered in February 2021.As for business in overseas nations, CellSeed entered into a business alliance with MetaTech (AP) Inc. (hereinafter referred to as MetaTech) in Taiwan in April of fiscal 2017 and the company submitted a clinical trial notification at the end of December 2018.
In January 2019, the advanced medical treatment for cartilage cell sheets, for which Tokai University Hospital had applied, was approved, and treatment for Regenerative Medicine B began in 2020.
Furthermore, CellSeed has licensed out the product to MetaTech and efforts are put forth to commercialize autologous cartilage sheets in accordance with the Taiwanese law (which is equivalent to those governing Japan’s Advanced Medical Care B Program).
Development of treatment methods using “cell sheet engineering”
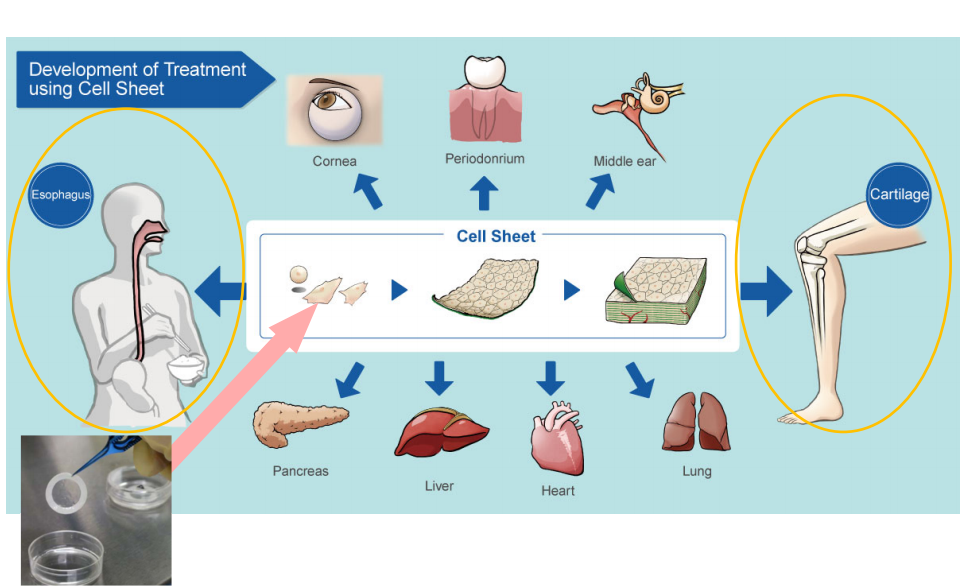
(From the company material)
“Epithelial Cell Sheet for Esophageal Regeneration”
About 26,300 patients within Japan are diagnosed with esophageal cancer every year with about 11,100 patients dying every year. The rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients. In addition, 90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma and five years comparative survival rates for males and females, which is said to be 41% and 46%, respectively, are under 50%. he endoscopic resection surgery (ESD) was posted in the drug price list from 2008 and is on the rise, but its side effect of esophageal stricture after surgery has been recognized as a problem.
However, introduction of epithelial cell sheets for esophageal regeneration will allow esophageal strictures to develop less frequently, which is expected to improve the quality of life of patients.
The treatment with “the epithelial cell sheet for esophageal regeneration” was developed by Tokyo Women's Medical University, in order to solve the problem with the regenerative medical treatment against esophageal cancer (treatment of esophageal tear and prevention of stenosis). Cells taken from the oral mucosa of a patient are cultured for about 2 weeks using the temperature-responsive cell cultureware to produce cell sheets. In conjunction with the process of culturing cell sheets, an endoscopic surgery for esophageal cancer excision is performed and the cell sheets are transplanted to the part of an esophageal tumor in the patient. Clinical studies were conducted at universities between 2008 and 2014: 10 cases at Tokyo Women's Medical University, 10 cases at Tokyo Women's Medical University and Nagasaki University (long-distance transport validation: cells collected at Nagasaki University were cultured at Tokyo Women's Medical University and transplanted at Nagasaki University), 10 cases at Karolinska University Hospital (Sweden), for a total of 30. The company signed a basic development agreement with Tokyo Women's Medical University and took over the university's research results for commercialization.
The company submitted a plan for clinical trials in April 2016 and finished the trials in March 2019, but failed to prove statistical superiority, and additional clinical trials became necessary. After consulting with PMDA about additional clinical trials, the company submitted a plan for additional clinical trials in October 2020, and the first case was registered in February 2021.
In February 2017, it received a designation under the “Sakigake Designation Scheme” for regenerative medicine products from the Ministry of Health, Labour and Welfare. The company plans to apply for an approval for manufacturing and sale in 2025.
Outside Japan, the company licensed MetaTech in Taiwan, with which it formed an alliance in April 2017, to use the sheet. In 2018, MetaTech submitted a plan for clinical trials.
Meanwhile, the company was proceeding with development in Europe, based on a subsidiary in Sweden, but it decided to stop the development in 2020, because endoscopic therapy had not been diffused in Europe as expected and the company had to concentrate on the acquisition of approval for production and sale in Japan.
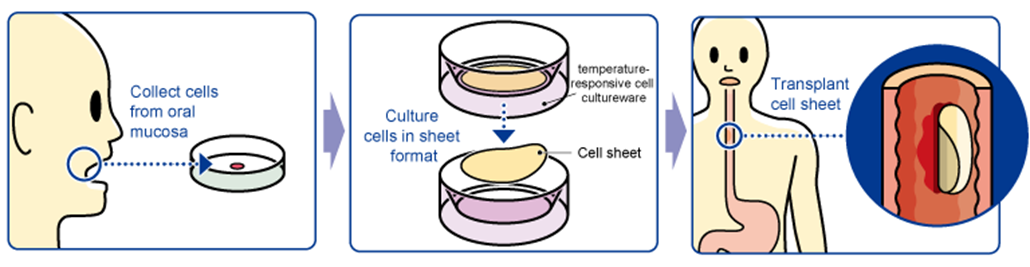
(From the company material)
“Regenerated Cell Sheet”
The company researched the “regenerated cell sheet” with Professor Masato Sato of Department of Orthopedics, Tokai University. Its indications are cartilage defects and osteoarthritis caused by sport injury and aging.
Knee osteoarthritis is slowly progressing, intractable degeneration of articular cartilage with no fundamental treatment. The number of potential patients in Japan is estimated to be about 30 million, of which about 10 million patients are thought to have subjective symptoms. Furthermore, population aging in Japan is expected to raise the number of patients diagnosed with the illness, making it a disease that needs to be dealt with immediately from the perspective of citizens’ healthy life expectancy and costs of long-term care and medical services. As of now, there are no methods to cure the injury completely, but the collaborative research with Professor Masato Sato is aimed at regenerating the cartilage surface radically. The cartilage of the knee is called hyaline cartilage, which is hard and excellent in cushioning and abrasion resistance properties, differing from the cartilages of the ear, nose, etc., and it is difficult to regenerate. However, it was confirmed in clinical research that the “regenerated cartilage sheet,” which is being researched collaboratively with the professor, can regenerate the cartilage of the knee as hyaline cartilage.
Professor Masato Sato started clinical research into autologous cartilage sheets in 2010 and has completed the study of 8 cases. In January 2019, “the cartilage regeneration treatment with autologous cell sheets” proposed by Tokai University Hospital was approved as Advanced Medicine B at “the 71st advanced medical care meeting” hosted by the Ministry of Health, Labor and Welfare.
CellSeed will manufacture cartilage cell sheets on consignment for treating patients by regenerating cartilage using autologous cell sheets as an advanced medical care program, and a surgery of the first patient under the category of Advanced Medical Care B Program was completed in August 2020.
The licensee MetaTech in Taiwan started the commercialization of autologous cartilage sheets in accordance with the Taiwanese law (complying with Advanced Medicine B in Japan), and performed transplantation for 10 patients. Then, CellSeed received a milestone revenue of 50 million yen.
In addition, in November of 2019, CellSeed and Tokai University jointly applied for a patent of “Cell Culture Sheet and Manufacturing Method and Use Thereof,” one of the outcomes of the joint research with Professor Masato Sato of the university, in the U.S. and the patent application was approved. This ensures that the intellectual property right of the product is now protected in Japan, the United States of America, and Europe.
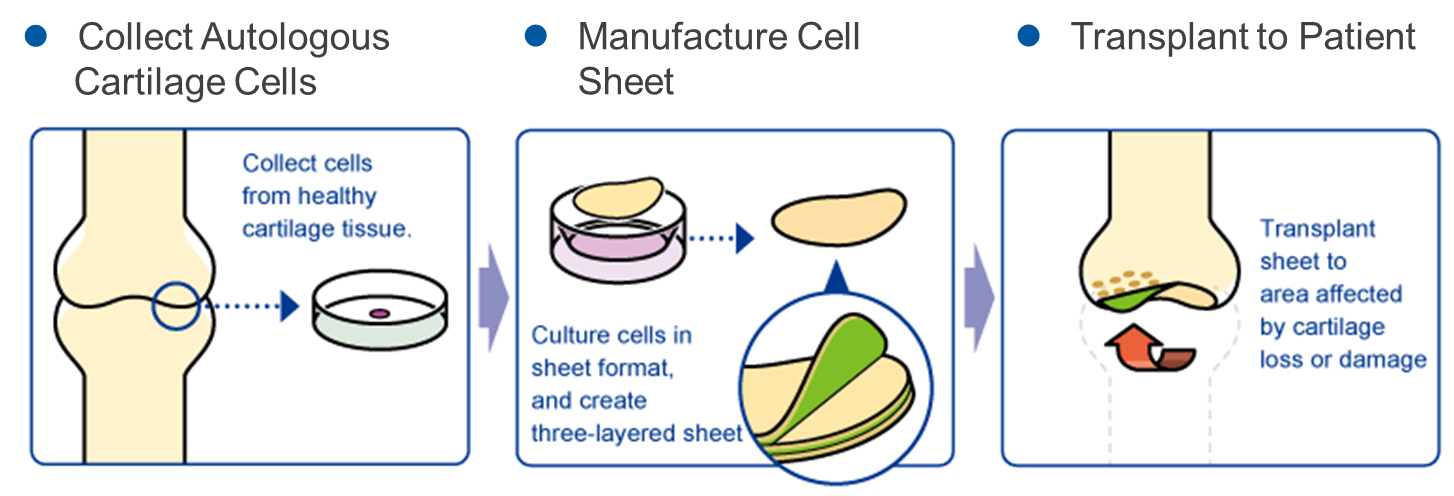
(Source: the company)
The company is also proceeding with the R&D of treatments with allogeneic cartilage sheets, which are derived from cells of people other than patients.
The professor Masato Sato started clinical research regarding the transplantation of allogeneic cartilage cell sheets in February 2017, and performed transplantation for 10 patients in 3 years. In December 2019, the 10th transplantation was finished.
The treatment with allogeneic cartilage sheets has been adopted in the project for developing evaluation methods, etc. for the industrialization of regenerative medicine (support for acceleration of development of regenerative medicine seeds) of AMED (project period: Oct. 2018 to Mar. 2021(plan)).
For developing cartilage cell sheets with allogeneic cells, the discarded tissue of patients with polydactyly who have six fingers, so it is necessary to solve ethical issues, but in December 2020, the company obtained approval for the provision of cartilage tissue collected from patients with polydactyly from National Center for Child Health and Development. In addition, in August 2022, the company reached an agreement with the center on the basic terms of a contract to establish a system for continuous provision of resected tissue during polydactyly (toe) surgery and commercialization for industrial use. Thus, it will be possible to continuously receive human tissue supply for clinical trials to manufacture and sell allogeneic cartilage cell sheets.
In addition, in July 2021, CellSeed's R&D proposal was selected by the Japan Agency for Medical Research and Development (AMED) as a subsidized project for the development of basic technologies for the industrialization of regenerative medicine and gene therapy (project to promote the industrialization of regenerative medicine, cell therapy, and gene therapy) in 2021.
In April 2022, a patent was granted and registered in Japan for a "tissue regeneration cultured cell sheet, its manufacturing method, and how to use it."
On the other hand, it is essential to confirm safety and efficacy and establish a system to ensure quality before clinical trials start. Thus, the company is also working on ensuring the safety and effectiveness of cell banks created using tissues, quality control of cell sheets, and establishing transportation methods.
Due to this sequence, research and development for clinical trials and manufacturing and sales approval are accelerating, and the company plans to submit a clinical trial notification at the end of 2022.
<Allogeneic cells> Development of cartilage cell sheets
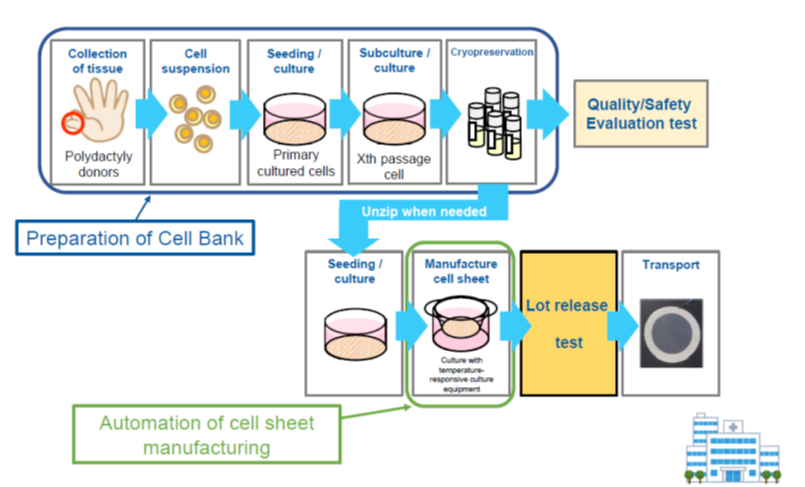
(From the company material)
In November 2020, the company entered into a technology transfer agreement with KanonCure Inc., a venture company originating from Tottori University, for the manufacturing of clinical trial cell sheets for treating liver diseases using mesenchymal stem cells, which KanonCure Inc. is developing as a regenerative medicine product. However, in response to a change in the development policy of KanonCure Inc., the company was notified of its intention to discontinue the technology transfer to CellSeed. After careful discussions between the two companies, the company agreed to terminate the agreement in December 2021.
(2) Regenerative Medicine Consignment Services
The company provides regenerative medicine services on consignment in relation to temperature responsive cell cultureware, etc., including development, manufacture, and sales and cell sheet products, including development of manufacturing methods and contract manufacturing, facility management and application support, and training and education in cell culturing technology such as 「UpCell®」「RepCell®」「HydroCell®」.
(1) Description of each business
*Development, manufacturing, and sales of cell cultureware, etc.
The temperature-responsive cell cultureware invented by Professor Okano of Tokyo Women's Medical University in 1989 can detach cells simply by lowering the temperature, making it possible to collect intact cell sheets for the first time in the world.
Temperature-responsive cell cultureware is sold worldwide, and research and development of treatment methods using cell sheets are being actively pursued by many researchers.
Until now, the company has developed and supplied various equipment products according to user needs, and in October 2022, the company plans to sell new products, UpCell® flasks and UpCell® 6-well cell culture inserts.
UpCell® is a device that can collect intact cells in a sheet without using enzymes that damage cells by fixing a temperature-responsive polymer to the surface of the device.
The UpCell® flask type will also be available for sale. The UpCell® flask type has a larger culture area than the conventional UpCell® dish, enabling the collection of larger amounts of undamaged cells, which will be optimal in research related to immunology and cell therapy. It is possible to recover cell sheets that maintain a higher level of biological functions through cell culturing that is close to the biological environment, such as co-culturing using the UpCell® 6-well cell culture inserts.
In the commissioned manufacturing of cell sheet products, the company develops and manufactures mainly cell sheets on consignment for pharmaceutical companies and research institutions. The company has a number of staff members with extensive knowledge and experience with cell culturing practices, such as clinical cultivatists certified by the Japanese Society for Regenerative Medicine, and they do those services at the facility with permission for manufacturing and processing specified cell products.
In addition, support is provided for the preparation of regulatory approval applications, obtaining manufacturing and marketing business licenses as well as training of engineers, for each stage from product development to manufacturing and sale.
The company's main commissioned projects for regenerative medicine contract service include the development of autologous cartilage cell sheets, cell sheets for treatment of liver disease, periodontal ligament cell sheets and pediatric autologous epithelial cell sheet etc.
Autologous cartilage cell sheets were approved in January 2019 as Advanced Medical Care B under the Act on the Safety of Regenerative Medicine, following which Tokai University started Advanced Medical Care B in 2020. The commissioned manufacturing of the autologous cartilage cell sheets by the CellSeed started. The contract was continued in 2022.
As described above, in November 2020, the company concluded a technology transfer agreement with KanonCure Inc., a venture company originating from Tottori University, for the manufacturing of clinical trial products for cell sheets for the treatment of liver diseases. However, following KanonCure Inc.'s notification of its intention to discontinue the technology transfer to CellSeed due to a change in its development policy, the two companies carefully discussed and agreed to terminate the agreement in December 2021.
The periodontal ligament cell sheet is the first project for commissioned manufacturing of cell sheets for use in physician-led clinical trials.
Pediatric autologous epithelial cell sheets are for children after surgery for congenital esophageal atresia.
In recent years, there has been a great deal of interest in the use of cells cultured in large quantities to produce biopharmaceuticals, conduct immunotherapy using the cells themselves, and even solve food and environmental problems.
When using proteolytic enzymes as a common cell retrieval technique, the cells are retrieved in a damaged state, making it difficult to fully maintain their original functions and components. In contrast, with the company's products, cells can be retrieved intact, and all the functions and components of the cells can be used while retaining their original properties, which is expected to greatly improve the efficiency and effectiveness of the industry in new markets.
In addition, in order to provide consistent quality and services, the company maintains its ISO09001 and ISO13485 certifications, and has obtained a manufacturing license for specified cell products and a manufacturing license for regenerative medicine products.
(2) Main facilities and equipment
Cell Culture Center
The cell sheets used for advanced medicine are cultivated at the cell culture center of CellSeed on commission.
With a floor space of about 763 square meters, the Cell Culture Center is equipped with an automated monitoring system that controls the cleanliness, room pressure, temperature and humidity, and operational status of equipment (such as incubators and reagent stockers), and a surveillance camera system throughout the entire facility. Besides, the facility is only twenty-minute drive from Haneda International Airport, making it possible and easy to transport products by air.
The permit to manufacture specific cell products based on the provisions of Article 35, Paragraph 1 of the Act on Ensuring Safety of Regenerative Medicine acquired in March 2017 (Licensing authority: Ministry of Health, Labor and Welfare) was renewed in March 2022. Under this license, the commissioned manufacturing of specific cell products is also possible.
In October 2018, the company obtained a license to manufacture regenerative medicine products and has built a commissioned manufacturing system for cell sheets that puts quality first.

(From the company material)
Aomi Cell Culture Innovation Center
The full-scale operation started in September 2021. The company develops and manufactures cell culture equipment, including laboratory flask products.
[1-4 Growth Strategy]
The company's two main growth strategies are “Business expansion of cell culture equipment” and “Promotion of business cooperation for global development.”
(1) Business expansion of cell culture equipment
In 1989, Professor Okano of Tokyo Women's Medical University invented temperature-responsive cell culture equipment that can exfoliate cells simply by lowering temperature as described above, making it possible to recover intact cell sheets for the first time, leading to the advancement of research and development of treatment methods using the cell sheet by many researchers.
In 2020, the company’s equipment business exceeded 100 million yen in sales for the first time. In September 2021, the company established a new development and manufacturing facility exclusively for cell culture equipment products, and also agreed to extend the sales contract with Thermo Fisher Scientific of the United States, an alliance partner for expanding sales of equipment products overseas, until 2025.
In recent years, many efforts have been made to manufacture biopharmaceuticals using cells cultured in large quantities, perform immunotherapy using the cells themselves, and solve food and environmental problems.
However, with proteolytic enzymes, that is, a commonly used cell recovery technique, cells are recovered in a damaged state, and it is difficult to completely maintain the original functions and components of the cells. On the other hand, by adopting this product, it is possible to recover cells without damage, and it is expected to greatly contribute to improvement of industrial efficiency and effectiveness in the new market, because all functions and components of cells are maintained.
In line with the steady expansion of product sales for the R&D phase for application to regenerative medicine, sales of products for new applications for mass culture of research cells are expanding rapidly, particularly overseas.
For this reason, the company is focusing on the development of products to provide solutions that meet the needs of new markets, such as the development of new cell culture equipment and the establishment of new manufacturing facilities, in addition to the conventional development of products in the regenerative medicine market.
The company is strengthening its sales structure to further expand its overseas sales channels. As mentioned above, the company extended its sales contract with Thermo Fisher Scientific, the company's alliance partner for expanding sales of equipment products overseas, and further strengthened their cooperation. In addition, the company established a quality management system to provide consistent quality and services and further enhance customer satisfaction. In January 2020, the company acquired ISO9001:2015 certification, an international standard.
(2) Promotion of business cooperation for global development
Aiming for global expansion, the company has been promoting business alliances by participating in exhibitions held not only in Japan but also in other Asian countries and Europe, such as an alliance with MetaTech in Taiwan in April 2017, establishment of Up Cell Biomedical Inc. in January 2020, and a presentation at “Translate! 2021 – Metrics and Milestones of Success” held in Berlin in January 2021. The company aims to find business partners by participating in exhibitions held in various regions.
2. Second quarter of the Fiscal Year ending December 2022 Earnings Results
[2-1 Non-Consolidated Earnings]
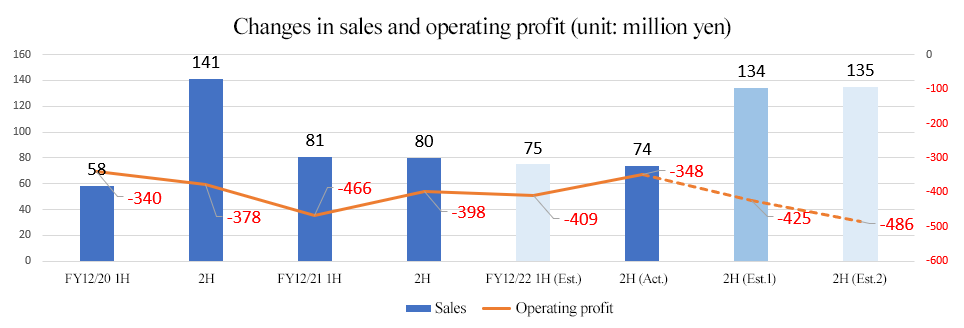
| FY12/21 2Q | FY12/22 2Q | YoY | Forecast Ratio | ||
Sales | 81 | 74 | -6 | -8.6% | -0 | -0.5% |
Gross Income | 43 | 36 | -7 | -16.4% | - | - |
SG&A | 509 | 384 | -124 | -24.4% | - | - |
R&D | 319 | 200 | -119 | -37.3% | - | - |
Operating Profit | -466 | -348 | +117 | - | +60 | - |
Ordinary Profit | -477 | -352 | +124 | - | +56 | - |
Quarterly Net Income | -486 | -357 | +129 | - | +53 | - |
* Unit: million yen. Quarterly Net profit is the profit attributable to owners of parent. Consolidated in 2Q of FY21/12. YoY are reference values calculated by Investment Bridge.
Sales decreased, operating loss shrink
Sales were 74 million yen, down 8.6% year on year.
The company posted an operating loss of 348 million yen (466 million yen in the previous term).
Both sales and profits were largely in line with forecasts.
[2-2 Segment trends]
| FY12/21 2Q | FY12/22 2Q | Increase/ decrease |
Regenerative medicine supporting business | 76 | 70 | -6 |
Cell sheet regenerative medicine business | 5 | 4 | -0 |
Consolidated sales | 81 | 74 | -6 |
Regenerative medicine supporting business | -19 | -40 | -21 |
Cell sheet regenerative medicine business | -319 | -207 | +111 |
Adjustments | -127 | -100 | +27 |
Consolidated operating Income | -466 | -348 | +117 |
*Unit: million yen Consolidated in 2Q of FY21/12. YoY are reference values calculated by Investment Bridge.
Regenerative medicine supporting business
Sales were 70 million yen, and operating loss was 40 million yen (19 million yen in the previous term).
The company has newly established a development and manufacturing facility for cell cultureware and started full-scale operation in September 2021. With the full-scale operation of this facility, the company will not only sell cell cultureware in the regenerative medicine market, but also sell cell cultureware products for new applications for culturing substantial amounts of research cells and mainly respond to solid overseas demand.
In addition, in the commissioned regenerative medicine business which supports regenerative medicine by utilizing the Cell Culture Center, the manufacturing of autologous cartilage cell sheets for advanced medical treatment was entrusted by Tokai University, which is a joint research partner, and the sales for 1 case was recorded in this fiscal year.
Cell sheet regenerative medicine business
Sales were 4 million yen, and operating loss was 207 million yen (319 million yen in the previous term).
In the pipeline of epithelial cell sheets for esophageal regeneration, additional clinical trials have been ongoing since October 20, 2020, when the clinical trial plan was submitted, and the time of submission of the manufacturing and marketing approval application is scheduled for 2025.
Although overseas expansion has been delayed due to the spread of COVID-19, the company will continue to provide support to MetaTech for businesses of the epithelial cell sheets for esophageal regeneration and cartilage cell sheets. It will also actively negotiate with potential new business partners outside Taiwan.
[2-3 Financial Condition and Cash Flows (CF)]
Summary of BS
| December 21 | June 22 | Increase/ decrease |
| December 21 | June 22 | Increase/ decrease |
Current Assets | 1,007 | 1298 | +290 | Current Liabilities | 171 | 207 | +36 |
Cash | 846 | 1168 | +321 | Accounts payable | 5 | 10 | +5 |
Receivables | 28 | 26 | -2 | Fixed Liabilities | 192 | 187 | -4 |
Inventories | 41 | 62 | +20 | LT Borrowings | 158 | 154 | -4 |
Fixed assets | 400 | 397 | -3 | Net Liabilities | 363 | 395 | +32 |
Total Assets | 1,408 | 1,695 | +287 | Net Assets | 1,044 | 1299 | +254 |
|
|
|
| Total Liabilities and Net Assets | 1,408 | 1,695 | +287 |
* Unit: million yen
*This figure is created by Investment Bridge Co., Ltd. Based on disclosed materials.
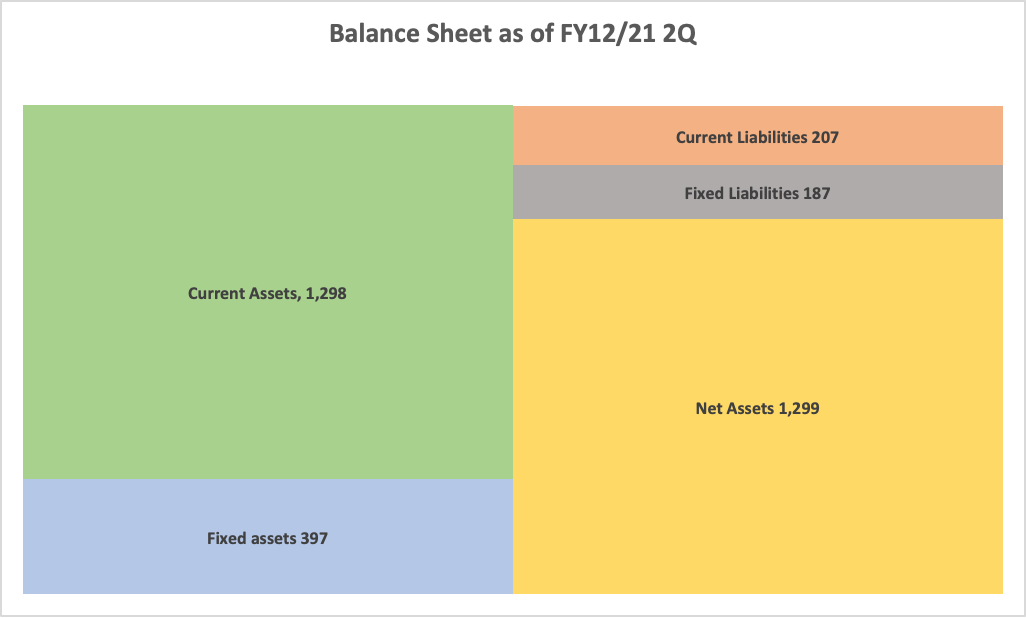
Total assets stood at 1,695 million yen due to the decrease of cash and deposit, increase 287 million yen from the end of the previous term. Net assets stood at 1,299 million yen, increase 254 million yen from the end of the previous term.
The capital adequacy ratio increased 2.7 percentage points from the end of the previous fiscal year to 75.1%.
Due to the exercise of the 22nd share acquisition right (issued in January 2022) between April 1, 2022, and June 22, 2022, capital increased 305 million yen.
[2-4 Topics]
◎ Exercising the 22nd share acquisition right
On June 22, 2022, all the 22nd share acquisition right issued on January 5, 2022, allotted to Barclays Bank PLC, were exercised. The total exercise price was approximately 610 million yen.
◎Agreed on the basic terms of the specimen provision contract for industrial use
In August 2022, it was announced that the company and the National Research and Development Agency National Center for Child Health and Development reached an agreement on the basic terms of a specimen provision contract concerning establishing a system for continuous supply and commercialization of resected tissue during polydactyly (toe) surgery for industrial use.
As mentioned in the section “Business Description regarding the Cartilage Cell Sheet,” in December 2020, the company obtained approval from the Ethics Committee of the National Center for Child Health and Development concerning the provision of cartilage tissue collected from polydactyly patients, making it possible to obtain available cartilage tissue that can be used industrially. With this agreement, it will now be possible to continue receiving the supply of human tissue for clinical trials, to manufacture and sell allogeneic cartilage cell sheets.
The cells isolated from the polydactyly (toe) tissue provided by the National Center for Child Health and Development might be provided to companies that use them to develop regenerated cartilage sheets and other products under regulations related to regenerative medicine products in various countries, including overseas, obtain manufacturing and selling approvals, and manufacture and sell them.
Facing an aging society, the company aims to contribute to treating knee osteoarthritis with allogeneic cartilage cell sheets and plans to proceed with preparations to submit a clinical trial notification at the end of 2022.
◎ Collaborative research on new treatments for central nerve injury-related diseases
In June 2022, the company started joint research with Hokkaido University Graduate School of Medicine on new treatment methods for central nerve injury-related diseases using the company's cell sheet engineering.
In this joint research, Hokkaido University will provide its own autologous bone marrow-derived cells, and, with the company's technical cooperation, they will conduct non-clinical studies on the quality, safety, and efficacy of treatments for diseases related to central nervous system injury.
Hokkaido University has a method for purifying, isolating, and culturing mesenchymal stem cells from human bone marrow with high purity. In this joint research, they aim to put regenerative medicine into practical use by making cell sheets from bone marrow-derived mesenchymal stem cells purified by this method using the company’s regenerative medicine basic technology, cell sheet engineering.
This regenerative medicine aims to repair the injury by transplanting a cell sheet prepared by separating and culturing it from human bone marrow fluid to the injured part of the patient. Until now, it has been considered difficult to recover nerve function in cases of damage to the central nervous system, mainly bone marrow injury, because there was no fundamental treatment method.
3. Fiscal Year ending December 2022 Earnings Forecasts
[3-1 Earnings forecasts]
| FY12/21 Act. | FY12/22 Est. | YoY | Progress Rate |
Sales | 161 | 209 | +47 | 35.7% |
Operating Income | -864 | -834 | +30 | - |
Ordinary Income | -887 | -834 | +53 | - |
Net Income | -914 | -838 | +76 | - |
* Unit: million yen
No change in earnings forecast, increase in sales, narrowing of loss
There is no change in the earnings forecast. For the term ending December 2022, sales are expected to increase 47 million yen to 209 million yen, while operating loss is projected to decrease 30 million yen to 834 million yen.
In the regenerative medicine support business, the company will continue to expand sales of its equipment, especially overseas. The company plans to release its new product UpCell® Flask and Insert in October. In addition, the company will promote contract manufacturing for regenerative medicine to support R&D and commercialization of regenerative medicine through comprehensive support for regenerative medicine. The contract for manufacturing of autologous cartilage cell sheets is scheduled to record sales for multiple cases from the third quarter onward.
Through these efforts, the sales in this segment are expected to reach 209 million yen.
The cell sheet regenerative medicine business will continue to mainly promote the development of epithelial cell sheets for esophageal regeneration and allogeneic cartilage cell sheets. In addition, it will actively negotiate with potential new business partners in Europe and the United States other than Asia for the introduction of pipeline technologies.
[3-2 Significant Events Related to Going Concern]
The balance of cash on hand (cash and deposits) as of the end of previous consolidated fiscal year was 930 million yen, and the financial base has been stable.
On the business side, however, the company has not yet been able to show the path to the early commercialization of its first cell sheet regenerative medicine product, which is a significant issue in the cell sheet regenerative medicine business. As of the end of June 2022, the company considered that the current situation raises doubts regarding the continuation of operations.
In order to resolve this situation, the company will promote the development of epithelial cell sheets for esophageal regeneration and cartilage cell sheets, and aim to realize the early commercialization of the first cell sheet regenerative medicine product and acquire more earning opportunities through business partners.
4. Conclusions
The first half of the term ending December 2022 witnessed progress in the development of cell engineering sheets, reaching an agreement with the National Center for Child Health and Development on the basic terms of a contract to provide resected tissue during polydactyly (toe) surgery for industrial use and launching joint research on new treatments for central nerve injury-related diseases. We will continue to pay attention to the earnings generated by the new product, UpCell® flasks and inserts, schedule next year, and to whether the submission of the clinical trial notification for the allogeneic chondrocyte sheet, which is expected by the end of this year, would progress as planned.
<Reference1: Mid-term Management Plan>
The company announced the mid-term management plan for the 3 years from the term ending December 2021 to the term ending December 2023.
(1) Activities in each business
Business | Outline |
Cell sheet regenerative medicine business | *To start additional clinical trials for epithelial cell sheets for esophageal regeneration, and aim to apply for certification for manufacturing and sale in 2025. *To accelerate the acquisition of non-clinical data for submitting a plan for clinical trials for allogeneic cartilage cell sheets at the end of 2022 *To redevelop collaborative business with MetaTech and a joint venture in Taiwan, with the aim of increasing earning opportunities *To actively form business alliances for diffusing cell sheet engineering created in Japan, with the aim of increasing revenues |
Regenerative medicine supporting business | *To cement the cooperation with Thermo Fisher Scientific, Inc. with the aim of increasing overseas sales of devices *To expand business by developing and supplying devices for new markets for mass culture of cells for research *To enrich and expand production systems and capabilities to meet overseas increasing demand of devices to new markets, and aim to increase earning opportunities *To proceed with the businesses of development, contract manufacturing, and consulting, with the aim of increasing earning opportunities |
(Major points)
*Epithelial cell sheets for esophageal regeneration
The company plans to apply for the certification for manufacturing and sale in 2025. It will have discussions for shortening the period of each clinical trial by increasing facilities for clinical trials, etc.
*Cartilage cell sheets
In December 2020, the company obtained the approval of the ethical review committee of National Center for Child Health and Development for the provision of cartilage tissue collected from patients with polydactyly, and became able to stably get cartilage tissue that can be used for commercial purposes. The company will accelerate R&D for obtaining approval for clinical trials, manufacturing, and sale of allogeneic cartilage cell sheets, and plans to submit a plan for clinical trials by the end of 2022.
*Business tie-up
In addition to the milestone income in the previous term, the company is scheduled to receive several-percent royalties according to the sales of autologous cartilage regeneration sheets from MetaTech. The company will make efforts to form new business alliances and find licensees in Asian countries, especially China, but any contracts were not signed in 2021, due to the differences in regulations, etc. The company will keep striving to form business alliances and find licensees with the aim of expanding the cell sheet regenerative medicine business inside and outside Japan while improving its business value by developing pipelines.
*Regenerative medicine supporting business
The company will enhance the development of new devices considering customer needs and emerging demand. For sales, the company will collect and analyze information on sales, etc. provided by major Japanese distributors and conduct marketing cooperatively, for the purpose of promoting the sales of devices, mainly temperature-responsive cell culture devices, and strengthen cooperation for boosting not only domestic sales, but also overseas sales of mainly Thermo Fisher.
The company will strive to enrich and expand its production systems for supplying products to new markets for mass culture of cells for research and expanding overseas sales, while maintaining the stable supply of products.
*Commissioned regenerative medicine business
The company will operate the consulting business by utilizing a variety of know-how acquired through the manufacturing of cell sheets entrusted by universities, enterprises, etc., commissioned development, and the cell sheet regenerative medicine business, with the aim of increasing earning opportunities.
*Establishment of a joint venture in Taiwan
In established joint venture, based on the new technologies provided by Japanese or Taiwanese universities and research institutes, the company will conduct R&D of products and methods for regenerative medicine by applying cell sheet engineering, have discussions on indications and optimize manufacturing methods for commercialization. A technology developed by Professor Du Yuan Kun of E-Da Hospital is one of candidates. In the regenerative medicine supporting business, the company plans to offer consulting services for R&D and support the application for approval for manufacturing and sale, and earn sales by receiving fees for technical instructions, etc.
(2) Numerical goals
As mentioned above, the company had disclosed its target value for the term ending December 2022 as "1.4 billion yen in sales, 20 million yen in operating profit, 19 million yen in ordinary profit, and 10 million yen in net profit." However, due to the impact of COVID-19 and changes in management strategies in terms of technological licenses of the company's pipeline with overseas business partner candidates, it will be difficult for the company to achieve a net profit of 10 million yen, and a net loss is expected for the term ending December 2022.
The company has withdrawn its performance targets for the term ending December 2023 as it expects to continue to be affected to a certain extent by changes in the business environment. The company plans to make an announcement as soon as it becomes possible to calculate its earnings forecast.
<Reference2: Regarding Corporate Governance>
◎Organization type, and the composition of executive directors and auditors
Organization type | Company with audit and supervisory committee |
Directors (excluding audit and supervisory committee members) | 6 directors, including 4 external ones |
Auditors and supervisory committee members | 3 committee members, including 3 external ones |
◎Corporate Governance Report(Latest Update:March 29, 2022)
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people’s health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.
In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)>
CellSeed has stated, “Our company implements all the basic principles stipulated in the Corporate Governance Code as a TES Growth listed company.”
This report is not intended for soliciting or promoting investment activities or offering any advice on investment or the like, but for providing information only. The information included in this report was taken from sources considered reliable by our company. Our company will not guarantee the accuracy, integrity, or appropriateness of information or opinions in this report. Our company will not assume any responsibility for expenses, damages or the like arising out of the use of this report or information obtained from this report. All kinds of rights related to this report belong to Investment Bridge Co., Ltd. The contents, etc. of this report may be revised without notice. Please make an investment decision on your own judgment. Copyright(C) Investment Bridge Co., Ltd. All Rights Reserved. |


