Bridge Report:(7776)CellSeed the second quarter of the Fiscal Year ending December 2023
 Setsuko Hashimoto, President | CellSeed Inc.(7776) |
 |
Company Information
Market | TSE Growth Market |
Industry | Precision Instrument (Manufacturing) |
President | Setsuko Hashimoto |
HQ Address | Telecom Center Building, Aomi 2-5-10, Koto-ku, Tokyo |
Year-end | December |
Homepage |
Stock Information
Share Price | Number of shares issued (End of the Term) | Total market cap | ROE Act. | Trading Unit | |
¥409 | 28,385,419 shares | ¥11,609 million | -69.8% | 100 shares | |
DPS Est. | Dividend yield Est. | EPS Est. | PER Est, | BPS Act. | PBR Act. |
¥0.00 | - | ¥-31.26 | - | ¥47.26 | 8.7 x |
*Stock price as of closing on September 22, 2023. The values of Share Outstanding, DPS, EPS were taken from the brief report on financial results for the second quarter of the term ending December 2023. ROE, BPS are actual results for the previous term.
Consolidated Earnings Trend
Fiscal Year | Sales | Operating Profit | Current Profit | Net Profit | EPS | DPS |
December 2019 | 275 | -780 | -786 | -782 | -66.60 | 0.00 |
December 2020 | 199 | -719 | -744 | -783 | -55.31 | 0.00 |
December 2021 | 161 | -864 | -887 | -914 | -53.18 | 0.00 |
December 2022 | 126 | -743 | -754 | -759 | -36.31 | 0.00 |
December 2023 Est. | 200 | -840 | -840 | -845 | -31.26 | 0.00 |
* The estimates were provided by the company. Units: million yen and yen. Net income is net income attributable to parent company shareholders until December 2021. Consolidated until December 2021. Unconsolidated from December 2022.
This Bridge Report presents CellSeed Inc.’s earnings results for the second quarter of the Fiscal Year ending December 2023 and other financial details.
Table of Contents
Key Points
1. Company Overview
2. Second quarter of the Fiscal Year ending December 2023 Earnings Results
3. Fiscal Year ending December 2023 Earnings Forecasts
4. Conclusions
<Reference: Regarding Corporate Governance>
Key Points
- In the second quarter of the term ending December 2023, sales declined 7 million yen year on year to 66 million yen, and operating loss augmented 10 million yen year on year to 359 million yen. In order to promote the sale of devices, the company cemented the cooperation with existing distributors and carried out active sales promotion. Tokai University entrusted the company with the manufacturing of autologous cartilage cell sheets, and the company posted sales from one case in the cumulative second quarter.
- Sales fell below the initial forecast by 28 million yen. In the commissioned regenerative medicine business, the registration of patients’ cases fell behind schedule, so it is expected to be concentrated in the second half of the fiscal year. Regarding the sale of devices, overseas sales did not reach the initially assumed amount. All kinds of profits exceeded the forecasts, because development outsourcing expenses fell below the initial forecast and the company curtailed the expenses for R&D and manufacturing, and SGA thoroughly.
- In May 2023, the company announced that it would be entrusted by Ikegami General Hospital with the manufacturing of autologous cartilage cell sheet to be implanted for treating knee cartilage damage. From now on, the company plans to expand the commissioned regenerative medicine business by undertaking the project of manufacturing cell sheets to be used in the uninsured healthcare field.
- There is no revision to the earnings forecast. For the term ending December 2023, sales are expected to grow 73 million yen year on year to 200 million yen and operating loss is projected to augment 96 million yen year on year to 840 million yen.
- In the regenerative medicine supporting business, the company will strive to expand overseas sales, mainly for devices. Overseas sales, which were sluggish in the previous fiscal year, are expected to grow. Regarding the commissioned manufacturing of autologous cartilage cell sheets, the company posted the sales from only 3 cases in the previous fiscal year, and this fiscal year, the company will be entrusted by Tokai University with the project of manufacturing and concentrate on undertaking new projects from medical institutions, such as Ikegami General Hospital. Through them, the sales in this segment are expected to reach 200 million yen.
- In the cell sheet regenerative medicine business, the company will continue activities for applying for the approval for the manufacturing and sale of epithelial cell sheets for esophageal regeneration in 2025.
- Regarding allogeneic cartilage cell sheets, the company submitted the notification on a third-phase clinical trial to Pharmaceuticals and Medical Devices Agency (PMDA) on September 20, 2023. After PMDA conducts a 30-day examination of the notification on a plan for a clinical trial, the company plans to start the clinical trial. The registration of subjects is expected to be started at the beginning of next year, considering the ethical review at a facility for conducting the clinical trial.
- They submitted a notification on a third-phase clinical trial for allogeneic cartilage cell sheets, which attracted attention of investors. There is no revision to the earnings forecast for the term ending December 2023, but the negotiation with candidate alliance partners, which is being conducted based on a confidentiality contract, is expected to progress further. We would like to pay attention to the news releases of the company as well as the progress of their clinical trials.
1. Company Overview
[1-1 Regenerative medicine of CellSeed]
Regenerative medicine is a new kind of medicine for regenerating and curing lost, damaged or deteriorated tissues.
CellSeed uses the fundamental technologies of “cell sheet engineering” developed in Japan by Professor Okano of the Tokyo Women’s Medical University in its “cell sheet regenerative medicine” that employs “cell sheets” for the cell regenerative medicine business, and the regenerative medicine support business, where temperature responsive cell cultureware used to fabricate cell sheets are developed and sold and the regenerative medicine consignment services, which support for research and development and commercialization of regenerative medicine, is provided.
“Cell sheet engineering” – Basic Technologies for Regenerative Medicine
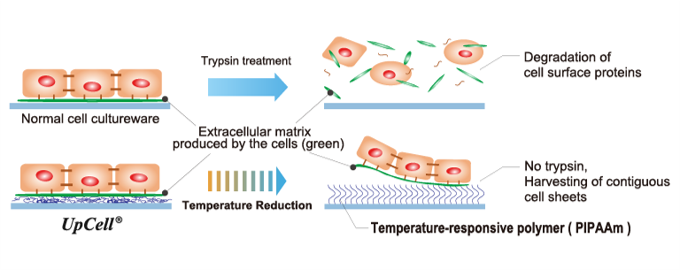
(From the company material)
"Cell sheet engineering" is a platform technology originating in Japan and the first of its kind in the world, which was invented by Mr. Teruo Okano, a professor emeritus of Tokyo Women's Medical University. Cells are cultured in “UpCell®,” a cell culture dish whose surface is processed with a temperature-responsive polymer that changes its molecular structure with temperature. The surface of a cell culture dish becomes moderately hydrophobic (water-repellent) at 37 °C, at which cells can attach, and hydrophilic (water-absorbent) at 20 °C, at which cells cannot attach. Therefore, by simply changing temperature, the organically bound "cell sheet" which retains the extracellular matrix (adhesion protein) can be recovered from the culture dish.
In general, cells secrete an extracellular matrix and grow by fixing themselves. In other words, cells cannot grow unless they are fixed somewhere while secreting adhesion proteins. However, in the conventional culture method, adhesion proteins are decomposed and recovered from cultured cells using proteolytic enzymes such as trypsin (there was no method for recovering cultured cells other than by decomposing adhesion proteins).
Huge Regenerative Medicine Market
The market size of regenerative medicine is expected to reach 2.5 trillion yen in Japan and 38 trillion yen worldwide in 2050, and a significant economic effect is expected.
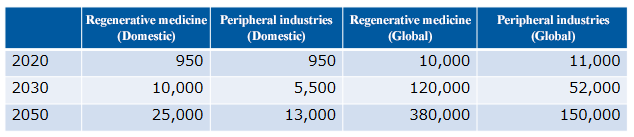
(From the company material, Unit: hundred million yen)
Expanding cell cultureware market
In recent years, along with progress in regenerative medicine research, there have been active efforts to manufacture biopharmaceuticals using substantial amounts of cultured cells, develop immunotherapy using cells, and solve food and environmental problems.
Currently, when using proteolytic enzymes, which is a commonly used cell collection technique, cells are collected in a damaged state, and it is difficult to completely maintain the original functions and components of cells. Introducing the company's temperature-responsive cell cultureware products makes it possible to collect cells without damage.
As a result, it is possible to use cells while maintaining all the functions and components that cells originally had. Thus, the company's products are attracting growing attention for their potential to significantly improve industrial efficiency and effectiveness in new markets.
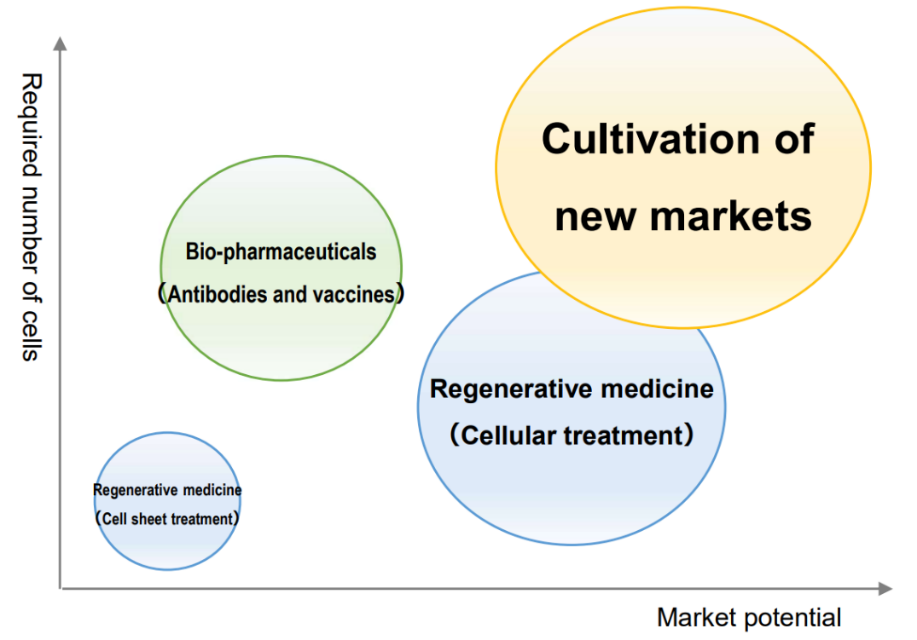
(From the company material)
(From the company material)
[1-2 Business model of CellSeed]
Mission: We take the initiative of contributing to global health care in the valuable and innovative field of regenerative medicine.
Using the outcomes of research into cell sheets conducted at universities as seeds, the company performs clinical trials, transforms them into regenerative medicine products, and provides products to patients.
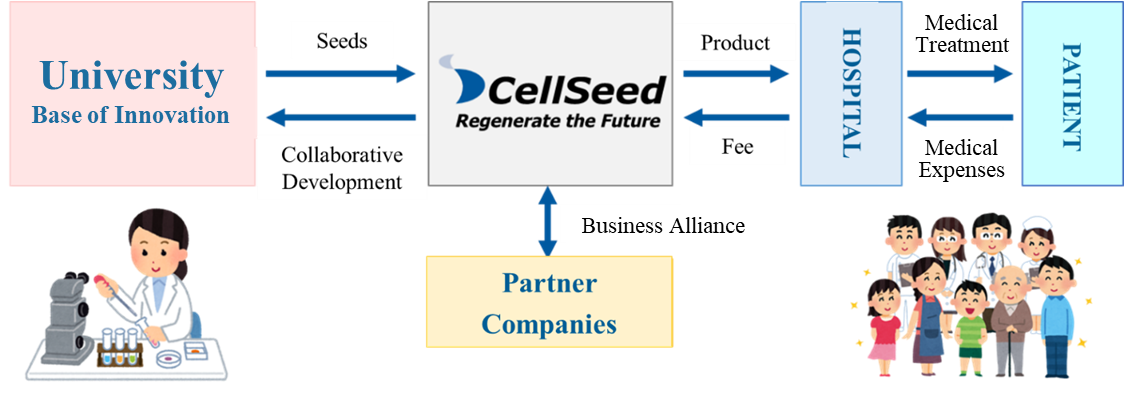
(From the company material)
[1-3 Business Description]
(1) Cell sheet regenerative medicine business
The development of treatments based on cell sheet engineering is used for various body parts. The company has also started joint research with Hokkaido University on new treatments for central nerve injury-related diseases while focusing on two products: the epithelial sheet for esophageal regeneration and the allogeneic cartilage cell sheet.
Development of treatment methods using “cell sheet engineering”
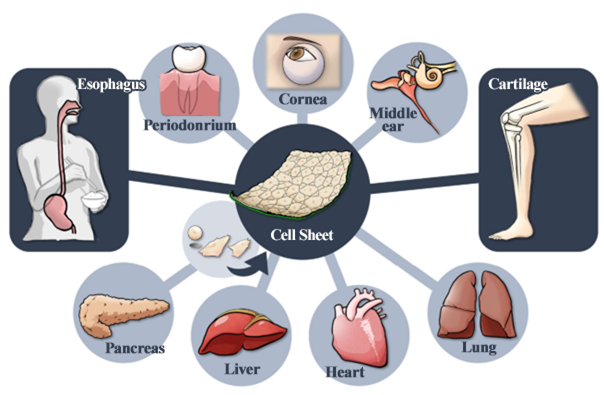
(From the company material)
“Epithelial Cell Sheet for Esophageal Regeneration”
About 26,300 patients within Japan are diagnosed with esophageal cancer every year with about 11,100 patients dying every year. The rate of occurrence and death related to esophageal cancer in male patients is five times that of female patients. In addition, 90% of the esophageal cancer cases diagnosed within Japan are squamous cell carcinoma and five years comparative survival rates for males and females, which is said to be 41% and 46%, respectively, are under 50%. he endoscopic resection surgery (ESD) was posted in the drug price list from 2008 and is on the rise, but its side effect of esophageal stricture after surgery has been recognized as a problem.
However, introduction of epithelial cell sheets for esophageal regeneration will allow esophageal strictures to develop less frequently, which is expected to improve the quality of life of patients.
The treatment with “the epithelial cell sheet for esophageal regeneration” was developed by Tokyo Women's Medical University, in order to solve the problem with the regenerative medical treatment against esophageal cancer (treatment of esophageal tear and prevention of stenosis). Cells taken from the oral mucosa of a patient are cultured for about 2 weeks using the temperature-responsive cell cultureware to produce cell sheets. In conjunction with the process of culturing cell sheets, an endoscopic surgery for esophageal cancer excision is performed and the cell sheets are transplanted to the part of an esophageal tumor in the patient. Clinical studies were conducted at universities between 2008 and 2014: 10 cases at Tokyo Women's Medical University, 10 cases at Tokyo Women's Medical University and Nagasaki University (long-distance transport validation: cells collected at Nagasaki University were cultured at Tokyo Women's Medical University and transplanted at Nagasaki University), 10 cases at Karolinska University Hospital (Sweden), for a total of 30. The company signed a basic development agreement with Tokyo Women's Medical University and took over the university's research results for commercialization.
The company submitted a plan for clinical trials in April 2016 and finished the trials in March 2019, but failed to prove statistical superiority, and additional clinical trials became necessary. After consulting with PMDA about additional clinical trials, the company submitted a plan for additional clinical trials in October 2020, and the first case was registered in February 2021.
In February 2017, it received a designation under the “Priority Review” for regenerative medicine products from the Ministry of Health, Labour and Welfare. The company plans to apply for an approval for manufacturing and sale in 2025.
Outside Japan, the company licensed MetaTech in Taiwan, with which it formed an alliance in April 2017, to use the sheet. In 2018, MetaTech submitted a plan for clinical trials.
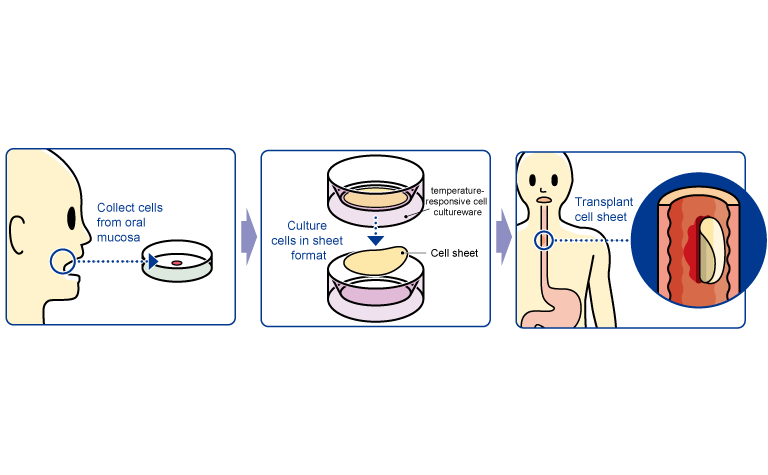
(From the company material)
“Allogeneic cartilage cell sheet”
The development of an allogeneic cartilage cell sheet, a cell sheet based on non-patient cells, is a joint research project with Professor Masato Sato, Department of Orthopedic Surgery, Tokai University, and it is indicated for cartilage defects and osteoarthritis caused by sports injuries and aging.
Knee osteoarthritis is slowly progressing, intractable degeneration of articular cartilage with no fundamental treatment. The number of potential patients in Japan is estimated to be about 30 million, of which about 10 million patients are thought to have subjective symptoms. Furthermore, population aging in Japan is expected to raise the number of patients diagnosed with the illness, making it a disease that needs to be dealt with immediately from the perspective of citizens’ healthy life expectancy and costs of long-term care and medical services. As of now, there are no methods to cure the injury completely, but the collaborative research with Professor Masato Sato is aimed at regenerating the cartilage surface radically. The cartilage of the knee is called hyaline cartilage, which is hard and excellent in cushioning and abrasion resistance properties, differing from the cartilages of the ear, nose, etc., and it is difficult to regenerate. However, it was confirmed in clinical research that the “allogeneic cartilage cell sheet,” which is being researched collaboratively with the professor, can regenerate the cartilage of the knee as hyaline cartilage.
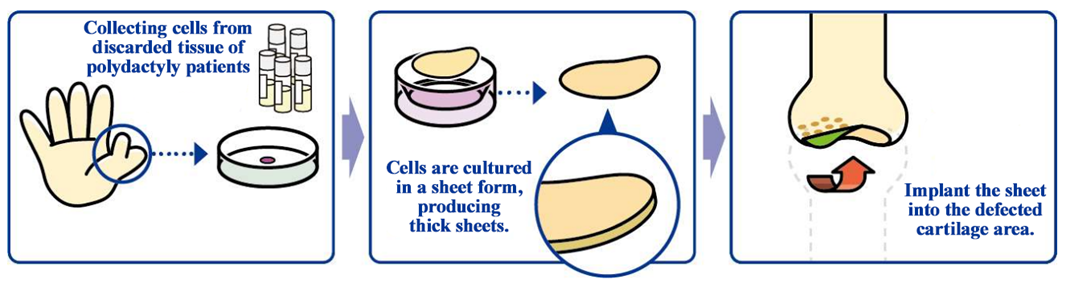
(Source: the cjompany)
Professor Masato Sato performed the world's first implant surgery in 2017, and 10 patients underwent implant surgery in the three years from 2017 to 2019.
The treatment with allogeneic cartilage sheets has been adopted in the project for developing evaluation methods, etc. for the industrialization of regenerative medicine (support for acceleration of development of regenerative medicine seeds) of AMED (project period: Oct. 2018 to Mar. 2021(plan)).
For developing cartilage cell sheets with allogeneic cells, the discarded tissue of patients with polydactyly who have six fingers, so it is necessary to solve ethical issues, but in December 2020, the company obtained approval for the provision of cartilage tissue collected from patients with polydactyly from National Center for Child Health and Development. In July 2021, a research and development project proposed by CellSeed was selected by the Japan Agency for Medical Research and Development (AMED) for the "Development of Basic Technologies for the Industrialization of Regenerative Medicine and Gene Therapy (Project to Promote the Industrialization of Regenerative/Cell Medicine and Gene Therapy)," which is a subsidy project (project period: August 2021-March 2023).
In addition, in July 2021, CellSeed's R&D proposal was selected by the Japan Agency for Medical Research and Development (AMED) as a subsidized project for the development of basic technologies for the industrialization of regenerative medicine and gene therapy (project to promote the industrialization of regenerative medicine, cell therapy, and gene therapy) in 2021.
In April 2022, a patent was granted and registered in Japan for a "tissue regeneration cultured cell sheet, its manufacturing method, and how to use it."
In January 2023, a research group led by Professor Masato Sato of Tokai University confirmed the safety and efficacy of allogeneic cartilage cell sheets implanted into cartilage defects in the knee joints one year after surgery in all 10 patients in a clinical study of knee osteoarthritis and their findings were published in the online journal npj Regenerative Medicine, a sister publication of Nature.
In addition, in March 2023, the company presented the results of the above-mentioned AMED project "Development of Basic Technologies for the Industrialization of Regenerative Medicine and Gene Therapy (Project to Promote the Industrialization of Regenerative/Cell Medicine and Gene Therapy)" at the Conference of the Japanese Society for Regenerative Medicine and is accumulating evidence of its effectiveness.
Meanwhile, before the start of clinical trials, the company is working to establish a system to ensure quality, by confirming safety as well as efficacy and establishing quality control and transportation methods for the cell sheets.
For cell banks made from tissues, a total of 20 tests, including cell count, viable cell rate, sterility test, endotoxin test, mycoplasma negative test, virus negative test, and other tests have been conducted to confirm its safety, and regarding cell sheets, 10 tests have been conducted including cell morphology, sheet properties, protein expression, virus negative test, and confirmation of the presence of chromosomal abnormalities.
Based on these results, the company planned to submit a notification of a clinical trial for the start of a Phase III trial (validation study) by the end of 2023, and on September 20, the notification was submitted to the Pharmaceuticals and Medical Devices Agency (PMDA) as scheduled. The study is expected to commence following a 30-day review of the notification of the clinical trial plan by PMDA. The enrollment of subjects is expected to start early next year, based on the ethical review at the site where the trial is being conducted.
(2) Regenerative Medicine Consignment Services
The regenerative medicine support business consists of the commissioned regenerative medicine business, which conducts the development of manufacturing methods, contract manufacturing, facility management, application support, and consulting for cell sheet products, and the device business, which develops, manufactures, and sells cell cultureware such as UpCell®, RepCell®, and HydroCell®.
①Description of each business
*Commissioned regenerative medicine business
The company is mainly engaged in commissioned development and manufacturing of cell sheets for pharmaceutical companies and research institutions. The company employs clinical culturists certified by the Japanese Society for Regenerative Medicine, and its staff with extensive culture experience develop manufacturing methods for regenerative medicine and other products and manufacture them at its cell culture center, which is licensed to manufacture specified cell processed products and regenerative medicine products.
In addition, the company provides support for preparing applications for regulatory approval, obtaining manufacturing and marketing licenses, and training engineers for all stages, from product development to manufacturing and marketing.
The company's main commissioned regenerative medicine services include periodontal ligament cell sheets, autologous cartilage cell sheets, pediatric autologous epithelial cell sheets, and cell sheet culture and detachment training.
Autologous cartilage cell sheets were approved in January 2019 as Advanced Medical Treatment B under the Law for Ensuring Regenerative Medicine Safety. Tokai University started Advanced Medical Treatment B in 2020. The commissioned manufacturing of autologous cartilage cell sheets by CellSeed has begun and will continue in 2022.
The periodontal ligament cell sheet is the first case of commissioned manufacturing of cell sheets for use in investigator-initiated clinical trials.
Pediatric autologous epithelial cell sheets are intended for children after surgery for congenital esophageal atresia.
In order to provide consistent quality and service, the company maintains its ISO09001 certification and has also obtained a license for manufacturing specific processed cell products and a license for manufacturing regenerative medicine products.
In May 2023, the company announced a new contract with Ikegami General Hospital to manufacture cell sheets for implantation of autologous cartilage cell sheets for knee joint cartilage damage.
As part of its regenerative medicine and other new medical treatment initiatives, Ikegami General Hospital will provide regenerative medicine to patients with damaged knee joint cartilage due to trauma or osteoarthritis by culturing their cartilage cells in sheet form and applying them to damaged cartilages in the knee joints to improve pain and joint function. Regenerative medicine treatment is provided at the expense of patients who are not eligible for the advanced medical treatment (cartilage regeneration therapy using autologous cell sheets) provided by Tokai University Hospital, as well as patients from overseas.
Ikegami General Hospital has submitted the provision plan necessary for the implementation of regenerative medicine to the Ministry of Health, Labor and Welfare, which has been accepted, and it is now preparing the patient acceptance system.
CellSeed expects to expand its commissioned manufacturing business by undertaking the manufacturing of cell sheets to be used in self-funded medical treatments.
*Equipment business
The temperature-responsive cell cultureware invented by Professor Okano of Tokyo Women's Medical University in 1989 can detach cells simply by lowering the temperature, making it possible to collect intact cell sheets for the first time in the world.
Temperature-responsive cell cultureware is sold worldwide, and research and development of treatment methods using cell sheets are being actively pursued by many researchers.
Until now, the company has developed and supplied various equipment products according to user needs such as universities, research institutes, and pharmaceutical companies, and in September 2022, the company starts to sell new products, UpCell® flasks and UpCell® 6-well cell culture inserts.
UpCell® is a device that can collect intact cells in a sheet without using enzymes that damage cells by fixing a temperature-responsive polymer to the surface of the device.
The UpCell® flask type will also be available for sale. The UpCell® flask type has a larger culture area than the conventional UpCell® dish, enabling the collection of larger amounts of undamaged cells, which will be optimal in research related to immunology and cell therapy. It is possible to recover cell sheets that maintain a higher level of biological functions through cell culturing that is close to the biological environment, such as co-culturing using the UpCell® 6-well cell culture inserts.
The company expects to meet new demand for the mass cultivation of cells for research purposes to develop preventive and therapeutic methods for various infectious diseases and cancer diseases and anticipates medium/long-term business growth.
In December 2022, the temperature-responsive cell culture device product "UpCell® ADVANCE" was registered in the Master Files for Devices (MAF) of the U.S. Food and Drug Administration (FDA).
MAF is a system under which a supply manufacturer registers its corporate information, manufacturing know-how, and other trade secrets and various data as MAF with the FDA in advance. This enables drug and medical device manufacturers to apply for marketing approval from the FDA simply by quoting the MAF number.
Although the completion of MAF registration does not necessarily mean that the FDA has finished confirming or evaluating the quality and safety of the product, the MAF registration is expected to contribute to the promotion of "UpCell® ADVANCE" because it will no longer be necessary for drug and medical device manufacturers to request from CellSeed to submit confidential information when they file applications with the FDA for products using "UpCell® ADVANCE."
In 2022, the company planned to start selling the UpCell® flasks, which have already been launched in Japan, outside Japan and to provide training for UpCell® users at the Aomi Cell Culture Innovation Center, which holds lectures about tips for actually preparing cell sheets and cell detachment.
(2) Main facilities and equipment
Cell Culture Center
The cell sheets used for advanced medicine are cultivated at the cell culture center of CellSeed on commission.
With a floor space of about 763 square meters, the Cell Culture Center is equipped with an automated monitoring system that controls the cleanliness, room pressure, temperature and humidity, and operational status of equipment (such as incubators and reagent stockers), and a surveillance camera system throughout the entire facility. Besides, the facility is only twenty-minute drive from Haneda International Airport, making it possible and easy to transport products by air.
The permit to manufacture specific cell products based on the provisions of Article 35, Paragraph 1 of the Act on Ensuring Safety of Regenerative Medicine acquired in March 2017 (Licensing authority: Ministry of Health, Labor and Welfare) was renewed in March 2022. Under this license, the commissioned manufacturing of specific cell products is also possible.
In October 2018, the company obtained a license to manufacture regenerative medicine products and has built a commissioned manufacturing system for cell sheets that puts quality first.

(From the company material)
Aomi Cell Culture Innovation Center
The full-scale operation started in September 2021. The company develops and manufactures cell culture equipment, including laboratory flask products, etc.
[1-4 Growth Strategy]
The company's two main growth strategies are “Business expansion of cell culture equipment” and “Promotion of business cooperation for global development.”
(1) Business expansion of cell culture equipment
In 1989, Professor Okano of Tokyo Women's Medical University invented temperature-responsive cell culture equipment that can exfoliate cells simply by lowering temperature as described above, making it possible to recover intact cell sheets for the first time, leading to the advancement of research and development of treatment methods using the cell sheet by many researchers.
In 2020, the company’s equipment business exceeded 100 million yen in sales for the first time. In September 2021, the company established a new development and manufacturing facility exclusively for cell culture equipment products, and also agreed to extend the sales contract with Thermo Fisher Scientific of the United States, an alliance partner for expanding sales of equipment products overseas, until 2025.
In recent years, many efforts have been made to manufacture biopharmaceuticals using cells cultured in large quantities, perform immunotherapy using the cells themselves, and solve food and environmental problems.
However, with proteolytic enzymes, that is, a commonly used cell recovery technique, cells are recovered in a damaged state, and it is difficult to completely maintain the original functions and components of the cells. On the other hand, by adopting this product, it is possible to recover cells without damage, and it is expected to greatly contribute to improvement of industrial efficiency and effectiveness in the new market, because all functions and components of cells are maintained.
(From the company material)
(2) Promotion of business cooperation for global development
In parallel with the steady expansion of product sales for the research and development phase aimed at application to regenerative medicine, product sales for new applications aimed at mass culturing of cells for research are increasing, mainly overseas. is rapidly expanding.
For this reason, the company is focusing not only on product development in the conventional regenerative medicine market, but also on product development to provide solutions that meet the needs of new markets, such as developing new cell culture equipment products and establishing new manufacturing facilities.
In December 2022, “UpCell® ADVANCE,” temperature-responsive cell cultureware, was registered in the Master Files (MAF) for Devices of Food and Drug Administration (FDA).
We are also strengthening our sales structure to further expand our overseas sales channels. As mentioned above, we have extended our sales contract with Thermo Fisher Scientific, our alliance partner for expanding sales of equipment products overseas, to further strengthen collaboration, provide consistent quality and service, and further enhance customer satisfaction. In order to achieve this goal, we have built a quality management system and obtained ISO9001:2015 certification, an international standard, in January 2020.
In addition, aiming for global expansion, the company has been promoting business alliances by participating in exhibitions held not only in Japan but also in other Asian countries and Europe, such as an alliance with MetaTech in Taiwan in April 2017, establishment of Up Cell Biomedical Inc. in January 2020, and a presentation at “Translate! 2021 – Metrics and Milestones of Success” held in Berlin in January 2021. The company aims to find business partners by participating in exhibitions held in various regions.
2. Second quarter of the Fiscal Year ending December 2023 Earnings Results
[2-1 Non-Consolidated Earnings]
| FY12/22 2Q | FY12/23 2Q | YoY | Forecast Ratio |
Sales | 74 | 66 | -7 | -28 |
Gross Income | 36 | 38 | +2 | - |
SG&A | 384 | 397 | +12 | - |
R&D | 200 | 233 | +33 | - |
Operating Profit | -348 | -359 | -10 | +90 |
Ordinary Profit | -352 | -366 | -13 | +83 |
Quarterly Net Income | -357 | -363 | -6 | +91 |
* Unit: million yen.
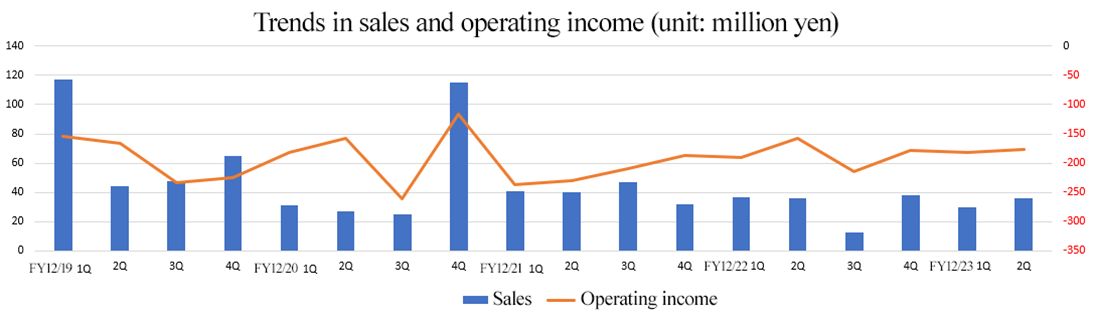
Sales decreased, operating loss expanded
Sales declined 7 million yen year on year to 66 million yen, and operating loss augmented 10 million yen year on year to 359 million yen. In order to promote the sale of devices, the company cemented the cooperation with existing distributors and carried out active sales promotion. Tokai University entrusted the company with the manufacturing of autologous cartilage cell sheets, and the company posted sales from one case in the cumulative second quarter.
Sales fell below the initial forecast by 28 million yen. In the commissioned regenerative medicine business, the registration of patients’ cases fell behind schedule, so it is expected to be concentrated in the second half of the fiscal year. Regarding the sale of devices, overseas sales did not reach the initially assumed amount. All kinds of profits exceeded the forecasts, because development outsourcing expenses fell below the initial forecast and the company curtailed the expenses for R&D and manufacturing, and SGA thoroughly.
[2-2 Segment trends]
| FY12/22 2Q | FY12/23 2Q | Increase/ decrease |
Regenerative medicine supporting business | 70 | 62 | -8 |
Cell sheet regenerative medicine business | 4 | 4 | +0 |
Consolidated sales | 74 | 66 | -7 |
Regenerative medicine supporting business | -40 | -27 | +12 |
Cell sheet regenerative medicine business | -207 | -235 | -27 |
Adjustments | -100 | -95 | +4 |
Consolidated operating Income | -348 | -359 | -10 |
* Unit: million yen.
Regenerative medicine supporting business
Sales were 62 million yen, and operating loss was 27 million yen (Sales were 70 million yen, and operating loss was 40 million yen in the previous term).
In the cell cultureware business, the company engaged in active sales promotion of devices, including the enhancement of cooperation with existing distributors and the setting of our booth at the 22nd Conference of the Japanese Society for Regenerative Medicine, which was held in March 2023, and the exhibition of the Japanese Association of Cancer Immunology, which was held in July. As mentioned above, overseas sales fell below the initial forecast.
In the commissioned regenerative medicine business, the company was entrusted by Tokai University, which is a joint research partner, with the manufacturing of autologous cartilage cell sheets for advanced medicine. Sales from one case were posted in the first quarter (Jan-Mar), and no sales were posted in the second quarter (Apr-Jun). Namely, sales from one case were posted in the cumulative second quarter.
Cell sheet regenerative medicine business
Sales were 4 million yen, and operating loss was 235 million yen (Sales were 4 million yen, and operating loss 270 million yen in the previous term).
Regarding allogeneic cartilage cell sheets, the company established a master cell bank, whose effectiveness and safety have been confirmed, for providing a raw material for producing allogeneic cartilage cell sheets to be used in clinical trials of enterprises, thanks to the progress of R&D, and presented its outcomes at the 22nd Conference of the Japanese Society for Regenerative Medicine.
In order to start a third-phase test (confirmatory trial) in Japan, they consulted with Pharmaceuticals and Medical Devices Agency (PMDA), and made preparations for submitting a notification on a clinical trial by the end of 2023. On September 20, the company submitted it to PMDA as scheduled. After PMDA conducts a 30-day examination of the notification on a plan for a clinical trial, the company plans to start the clinical trial. The registration of subjects is expected to be started at the beginning of next year, considering the ethical review at a facility for conducting the clinical trial.
In addition, the company strove to form business alliances and conclude joint research contracts with companies inside and outside Japan. In particular, they made proactive efforts to conclude contracts with candidate alliance partners, as the interest in allogeneic cartilage cell sheets has been recently growing, and have engaged in negotiations with candidate alliance partners in accordance with a confidentiality contract, in order to maximize the value of allogeneic cartilage cell sheet.
[2-3 Financial Condition and Cash Flows (CF)]
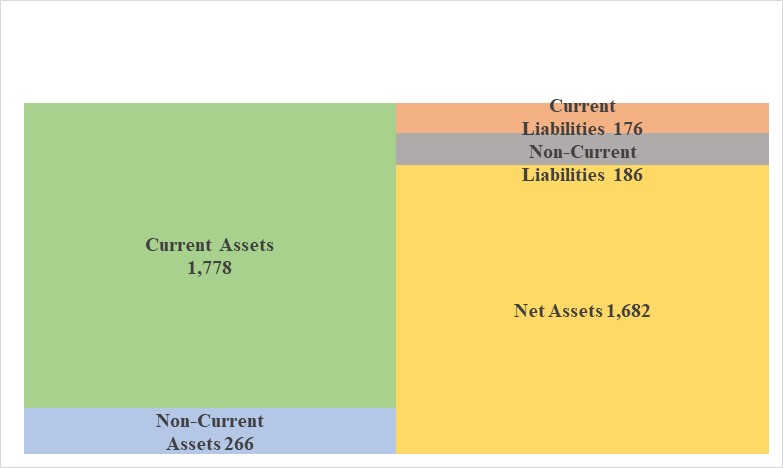
Summary of BS
| December 2022 | June 2023 | Increase/ decrease |
| December 2022 | June 2023 | Increase/ decrease |
Current Assets | 1,231 | 1,778 | +546 | Current Liabilities | 180 | 176 | -4 |
Cash | 1,072 | 1,589 | +517 | Accounts payable | 7 | 6 | +0 |
Receivables | 25 | 23 | -2 | Fixed Liabilities | 184 | 186 | +1 |
Inventories | 56 | 58 | +2 | LT Borrowings | 151 | 147 | -3 |
Fixed assets | 311 | 266 | -45 | Net Liabilities | 365 | 362 | -2 |
Total Assets | 1,543 | 2,045 | +501 | Net Assets | 1,178 | 1,682 | +504 |
* Unit: million yen |
|
|
| Total Liabilities and Net Assets | 1,543 | 2,045 | +501 |
*This figure is created by Investment Bridge Co., Ltd. Based on disclosed materials.
Total assets stood at 2,045 million yen due to the increase of cash and deposit, increase 510 million yen from the end of the previous term. Net assets stood at 1,682 million yen, due to the increase of capital and capital surplus ,and negative retained earnings, increase 504 million yen from the end of the previous term.
The capital adequacy ratio increased 6.3 percentage points from the end of the previous fiscal year to 81.1%.
[2-4 Topics]
◎Regarding some press reports on MetaTech (AP) Inc.
Some media in Taiwan reported that “the information on ‘cell sheet engineering’ of CellSeed was leaked from MetaTech, with which the company concluded contracts for exclusive development and distributorship in Taiwan regarding the cell sheet regenerative medicine business (epithelial cell sheets for esophageal regeneration and autologous cartilage cell sheets),” “MetaTech is researching and developing allogeneic cartilage cell sheets,” and “technologies for cartilage sheets were leaked to China.”
The company gave the following comments regarding these reports.
(Taken from the material disclosed by the company on July 26, 2023)
Our company provided MetaTech with the rights for “autologous cartilage cell sheets” and “sheets for esophageal regeneration” in Taiwan only, and did not offer any rights for “allogeneic cartilage cell sheets.”
Our company requested MetaTech to check the facts about these press reports, stop using leaked information, and recover it, but MetaTech replied that they cannot explain the details, because they are in the middle of judiciary proceedings.
Our company is investigating the facts, and discussing necessary legal actions against MetaTech, while consulting with corporate lawyers.
Regarding “allogeneic cartilage cell sheets,” in order to start a third-phase test (confirmatory trial) in Japan, we consulted with Pharmaceuticals and Medical Devices Agency (PMDA), and are steadily preparing for submitting a notification on a clinical trial (note: On September 20, we submitted a notification on a third-phase clinical trial for allogeneic cartilage cell sheets [CLS2901C]). Our company is negotiating with multiple enterprises inside and outside Japan as candidate alliance partners. Accordingly, we think that the direct damage due to information leakage, etc. is extremely limited, and will hardly affect the development of allogeneic cartilage cell sheets. If there emerges any item that would affect business performance and should be disclosed, we will disclose it swiftly.
Although the above problem made shareholders and other stakeholders worry, we will continue our efforts to improve our corporate value. We would appreciate your continued support.
◎Ikegami General Hospital of Medical Corporation Showakai entrusted the company with the manufacturing of cell sheets.
In May 2023, it was announced that the company would be entrusted by Ikegami General Hospital of Medical Corporation Showakai with the manufacturing of autologous cartilage cell sheets to be implanted for treating knee cartilage damage.
For details, see the section “Corporate Profile, Commissioned Regenerative Medicine Business.”
This project was taken into account when making the initial forecast.
3. Fiscal Year ending December 2023 Earnings Forecasts
[3-1 Earnings forecasts]
| FY12/22 Act. | FY12/23 Est. | YoY |
Sales | 126 | 200 | +73 |
Operating Income | -743 | -840 | -96 |
Ordinary Income | -754 | -840 | -85 |
Net Income | -759 | -845 | -85 |
* Unit: million yen
No change in earnings forecast, increase in sales, expanding of loss
There is no change in the earnings forecast. For the term ending December 2023, sales are expected to increase 73 million yen to 200 million yen, while operating loss is projected to increase 96 million yen to 840 million yen.
<Regenerative medicine supporting business>
The company will mainly keep expanding overseas sales of devices.
They will strive to make UpCell® flasks, which were released in September 2022, contribute to revenues, to grow business and improve corporate value in the medium/long term. The inventory adjustment of overseas distributors in the wake of the spread of COVID-19 subsided, and order receipt recovered, so sales are expected to grow in the term ending December 2023.
Regarding the commissioned manufacturing of autologous cartilage cell sheets, the company will keep undertaking the manufacturing project of Tokai University, and concentrate on receiving orders for the manufacturing from other medical institutions. Accordingly, the sales from multiple cases are expected to be posted from the third quarter.
In addition, through comprehensive support related to regenerative medicine, we will promote regenerative medicine contract manufacturing that supports research, development, and commercialization of regenerative medicine.
Through them, the sales in this segment are expected to reach 200 million yen.
<Cell sheet regenerative medicine business>
Regarding epithelial cell sheets for esophageal regeneration, the company will keep making preparations for applying for the approval for manufacturing and sale in 2025.
Regarding allogeneic cartilage cell sheets, the company submitted a notification on a clinical trial to Pharmaceuticals and Medical Devices Agency (PMDA) on September 20 as mentioned above. They will accelerate development further, for conducting clinical trials and obtaining the approval for manufacturing and sale.
We will continue to actively negotiate with potential new business partners to license out pipeline technology.
[3 -2 Significant Events Related to Going Concern]
The balance of cash on hand (cash and deposits) as of the end of previous consolidated fiscal year was 1,072 million yen, due to the procurement of funds through the exercise of stock acquisition rights, and the financial base has been stable.
On the business side, however, the company has not yet been able to show the path to the early commercialization of its first cell sheet regenerative medicine product, which is a significant issue in the cell sheet regenerative medicine business.
current fiscal year.
The company continues the following measures in order to improve this situation.
To seize earning opportunities by commercializing the first product for cell sheet-based regenerative medicine as soon as possible and promoting business alliance.
The company will proceed with the development of epithelial cell sheets for esophageal regeneration and allogeneic cartilage cell sheets, and try to seize earning opportunities by commercializing the first product for cell sheet-based regenerative medicine as soon as possible and finding business alliance partners, to improve the above situation.
4. Conclusions
They submitted a notification on a third-phase clinical trial for allogeneic cartilage cell sheets, which attracted attention of investors. There is no revision to the earnings forecast for the term ending December 2023, but the negotiation with candidate alliance partners, which is being conducted based on a confidentiality contract, is expected to progress further. We would like to pay attention to the news releases of the company as well as the progress of their clinical trials.
<Reference: Regarding Corporate Governance>
◎Organization type, and the composition of executive directors and auditors
Organization type | Company with audit and supervisory committee |
Directors (excluding audit and supervisory committee members) | 4directors, including 3 external ones(including an independent one) |
Auditors and supervisory committee members | 3 committee members, including 3 external ones (including an independent one) |
◎Corporate Governance Report(Latest Update:April 7, 2023)
Basic Policy
With the missions to introduce technological innovations, to exert creativity and to contribute to people’s health and welfare by providing high-quality products and services, we are enhancing corporate governance to raise quality in all of our corporate activities.
In the future, we will increase our accountability further to improve the transparency of disclosed information and strengthen our checking system even more.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)>
CellSeed has stated, “Our company implements all the basic principles stipulated in the Corporate Governance Code as a TES Growth listed company.”
This report is not intended for soliciting or promoting investment activities or offering any advice on investment or the like, but for providing information only. The information included in this report was taken from sources considered reliable by our company. Our company will not guarantee the accuracy, integrity, or appropriateness of information or opinions in this report. Our company will not assume any responsibility for expenses, damages or the like arising out of the use of this report or information obtained from this report. All kinds of rights related to this report belong to Investment Bridge Co., Ltd. The contents, etc. of this report may be revised without notice. Please make an investment decision on your own judgment. Copyright(C) Investment Bridge Co., Ltd. All Rights Reserved. |


