| Linical Co., Ltd. (2183) |
|
||||||||
Company |
Linical Co., Ltd. |
||
Code No. |
2183 |
||
Exchange |
Mothers Market, TSE |
||
Industry |
Service |
||
CEO |
Kazuhiro Hatano |
||
HQ Address |
10 Flr., Shin-Osaka Brick Building, 6-1 Miyahara 1-chome, Yodogawa-ku, Osaka, Japan |
||
Year-end |
March |
||
URL |
|||
* Stock price as of closing on November 9, 2012. Number of shares issued at the end of the most recent quarter excluding treasury shares.
|
||||||||||||||||||||||||
|
|
* Estimates are those of the Company.
We present this Bridge Report about Linical Co., Ltd. and details of its first half and full year March 2013 earnings and estimates. |
|
| Key Points |
 |
| Company Overview |
|
Linical' s main customers include Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, Limited, Eisai Company, Limited, Otsuka Pharmaceutical Company, Limited, Shionogi & Company, Limited, and primarily other Japanese pharmaceutical companies. Moreover, phase II clinical trials are conducted to test the safety, efficacy, usage, and dosage of pharmaceutical products. Phase III clinical trials take these results and confirm them in actual treatments to test for efficacy and safety. <CRO Business Service Description>
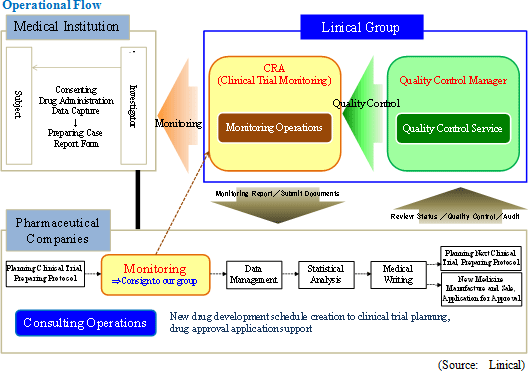
Linical' s business can be divided between the CRO and CSO segments and each accounted for 95.6% and 4.4% of sales respectively during fiscal year March 2012. The CRO business specializes in "monitoring operations" and also includes "quality control" and "consulting" operations.
Monitoring Service
Monitoring is conducted to ensure that planning and procedures are performed properly and comply with all laws. Monitoring is conducted by clinical research associates (CRA) by visiting the medical institution that is implementing the clinical trial and by explaining the study drug, protocol, and procedures, and monitoring the trial to collect data following the start of clinical trials.
Quality Control Service
CRAs use specific checklists to review data collected from the medical institutions.
Consulting Activities Service
Linical provides consulting services for client pharmaceutical companies that range from preparing schedules for new drugs under development to filing new-drug applications for approval. In addition, Linical' s technical support is designed to ensure that the development process progresses efficiently.
 <Corporate History>
Linical Co., Ltd. was created in June 2005 by nine members who used to work on development of immunosuppressant drugs at Fujisawa Pharmaceutical Co., Ltd. (Currently called Astellas Pharma Inc.). Initially, the newly formed Company focused efforts upon development of products in the realms of central nervous system (CNS) and oncology, and received an order for a project in the CNS realm from Otsuka Pharmaceutical Co., Ltd. Thereafter, staffing was fortified to strengthen order taking activities. Furthermore, Linical was able to scout staff with expertise in the realm of oncology who worked at foreign pharmaceutical companies, and they were able to expand near term orders. In January 2006, Linical acquired Aurora as a subsidiary as part of its efforts to enter the site management organization business (SMO). However in May 2007 Aurora was sold due to Linical' s decision to focus its business resources upon the CRO business. LINICAL USA, INC. was established in California, USA in July 2008 as a fully owned subsidiary to provide support services to Japanese pharmaceutical companies seeking to enter the United States market. In October 2008, Linical listed its shares on the Mothers Section of the Tokyo Stock Exchange. 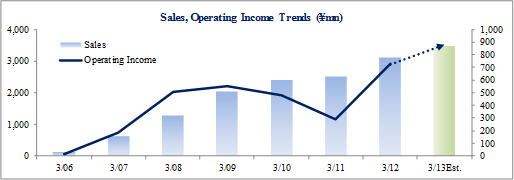 <Strengths and Market Environment>
The CRO industry saw a contraction in its market scale, and it underwent consolidation and restructuring in the aftermath of the Lehman Shock with a reduction in the number of companies operating within the industry. Currently the number of projects is increasing due to improvements in the business environment, but there are only a few surviving CRO companies that can take advantage of the recovery in demand for services in more difficult realms of CNS and oncology. And while competition is intensifying to gain access to human resources needed to capture this growing demand, the business environment overall remains favorable.
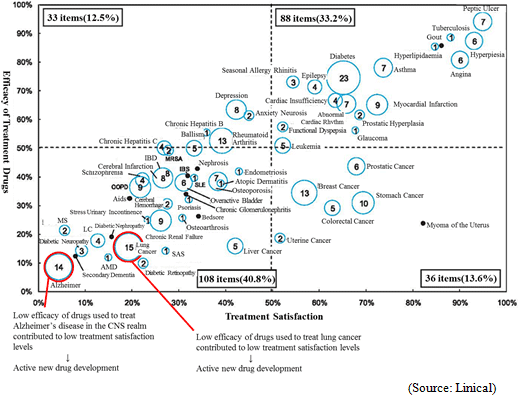
(1) Strengths in CNS, Oncology Monitoring, Highly Difficult Therapeutic with Few Competitors
One of Linical' s strengths is its specialization in monitoring services for highly difficult therapeutic areas of central nervous system and oncology, where there are few competitors. For example, in the realm of central nervous system diseases, evaluating the efficacy of drugs prescribed for patients afflicted with Alzheimer' s disease is highly difficult. At the same time in the realm of oncology, evaluating symptoms to determine whether they are the result of side effects of drugs or the cancer itself is difficult. Therefore high levels of responses and expertise in monitoring are necessary in these difficult realms. Similar to CNS and oncology realms, new drug development for patients of acute diseases and intractable diseases (Difficult diseases), which are also highly difficult therapeutic areas, is very active (There are only a few number of CROs that can respond to these situations). At the same time, evaluation of the efficacy of drugs for adult lifestyle related diseases are easier because conditions of patients undergoing clinical trials tend to be relatively stable.
  (2) High Profitability
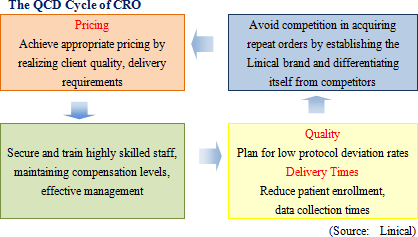
In addition to high levels of various task execution capabilities in the clinical work field for efficacy and safety confirmation, high levels of knowledge and technology in the overall CRO operations are also strengths of Linical. The protocol deviation rates on projects undertaken by Linical have been held to extremely low levels, and the implementation period, including the period required for patient enrollment and data collection, for about 80% of all projects has been shortened. Because of its high levels of service quality and quick delivery times in highly difficult therapeutic areas, Linical is able to book orders without having to offer reduced prices and is able to overcome the scale merit advantage of its larger competitors.
|
| Measures to Promote Growth |
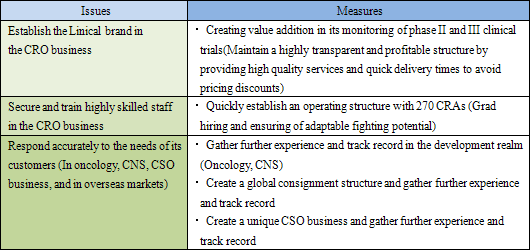 (1) Establish the Linical Brand in the CRO Business
As explained earlier, Linical has been successful in achieving extremely low protocol deviation rates on projects, and has been able to reduce the implementation period on close to 80% of its projects, evidence of its high quality levels and quick delivery capabilities. By endeavoring to maintain high quality and short delivery periods (High value addition) in the monitoring of phase II and III clinical trials, Linical is on course to firmly establishing the "Linical brand" and achieving appropriate pricing to maintain its high level of profitability.
 (2) Secure, Training Highly Skilled Staff in the CRO Business
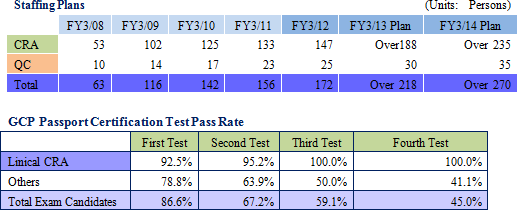
Currently, pricing on orders, which had fallen in the aftermath of the Lehman Shock, have begun to recover, but only Linical and a handful of other CROs are benefitting from this improvement because most of the new drug development activities have been focused upon applications in highly difficult therapeutic areas. At the same time, Linical and most of these other companies share the same problem of limitations in their capacity to provide services. Therefore Linical plans to increase its staff numbers by 20% through the hiring of new graduates (Hiring over 20% presents a risk of reducing quality levels) in addition to hiring similar levels of midcareer staff with experience, for which competition to hire is high. As of November 2012, Linical has 165 CRAs, and seeks to create a structure with a total of 300 staff including managers and quality control (QC) staff at an early stage (After other division staff are included, the total number of employees would reach 400).
 (3) Responding to Customers' Needs (CNS, Oncology, CSO Business, Overseas Markets)
① Responses in CNS and Oncology Realms
Linical' s efforts to fortify its responses to new drug development in the realms of CNS disease and oncology have led to successful results. Specifically, while standardization of efficacy evaluation for CNS realm diseases is difficult, Linical has focused upon this realm since its establishment and it boasts of managers and CRAs with strong experience in responding to the needs of customers. Furthermore, in the oncology realm many cases are critical in general nature and they require careful and speedy response in the safety information reporting process. Consequently, Linical is promoting responses that meet the growing needs of clients and leverage the skills of the Company' s experienced managers, while at the same time allowing them to gather further knowhow and experiences.
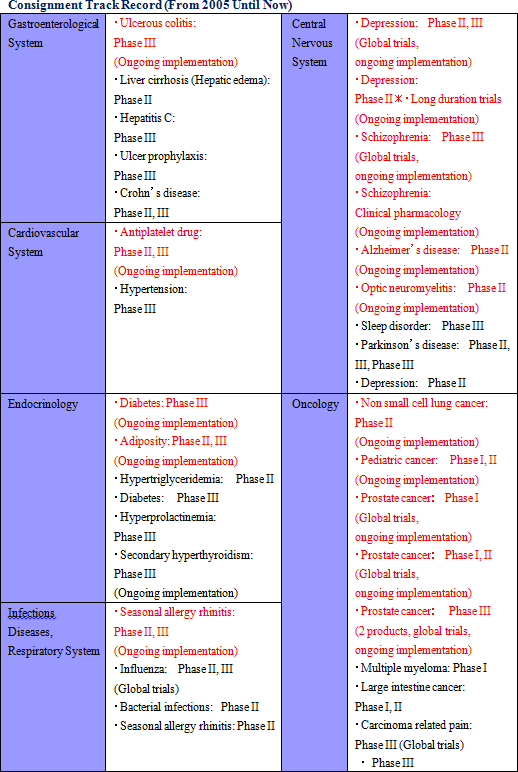 ② Overseas Market Responses (Global Development)
In order to respond to the anticipated growing demand for joint clinical trials on a global basis, Linical will promote a business strategy that focuses upon Asia, the United States and Europe, in addition to Japan. On July 15, 2008, LINICAL USA, INC. (California) was established to provide consulting services to medium sized Japanese pharmaceutical manufacturers seeking to enter overseas markets. With a view to providing consigned monitoring for clinical trials in the United States, a confidentiality agreement has been exchanged with the United States 9 CRO companies, which are medium sized companies providing services on a worldwide basis. Furthermore, Linical is gathering information on the CRO market in Europe, and is hiring staff from various parts of Asia in preparation for consigned Asian market monitoring services for which there is strong demand. While Linical will begin with global study support services, over the intermediate term they will cultivate core staff that can provide study services in the Asian market. In addition, the Company is also considering global M&A options as a means of establishing its global structure at an early stage.
③ CSO Business Responses
Contracted sales organization in general is the business where medical representatives (MR) are dispatched to client companies. Linical can leverage the knowhow of its CRO business and its specialized patient realms to increase orders for consigned product marketing services, and post market launch data planning and gathering (Not only simple human resources dispatching services, but a service where the responsibility for various tasks are also assumed.). During fiscal year March 2012, Linical was successful in capturing new orders in the CNS realm for product marketing.
 |
| First Half Fiscal Year March 2013 Earnings Results |
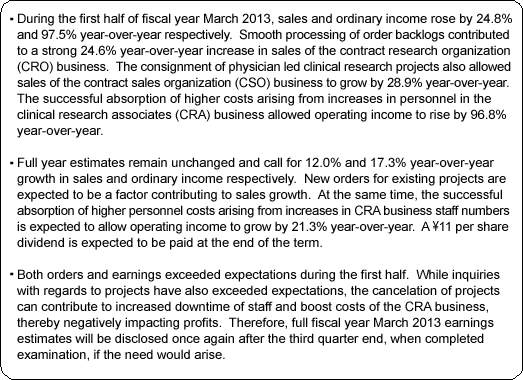
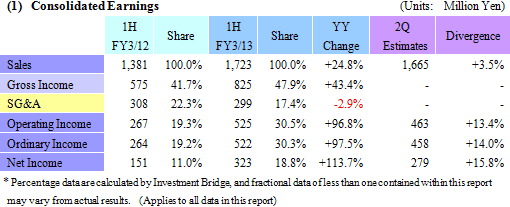 Sales, Ordinary Income Rise 24.8%, 97.5% Year-Over-Year
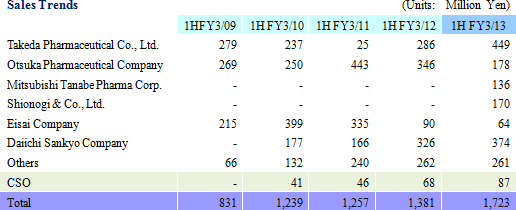
During the first half of the fiscal year, sales rose by 24.8% year-over-year to ¥1.723 billion. Disappearance of consigned project cancellations and smooth processing of order backlog allowed sales of the CRO business to grow. Physician led research project consignment also contributed to growth in sales of the CSO business. With regards to profits, high capacity utilization rates of CRAs allowed gross margin to improve by 6.2% points to 47.9%. At the same time, ongoing efforts to reduce costs succeeded in reducing sales, general and administrative costs by 2.9% year-over-year and allowed operating income to rise by 96.8% year-over-year to ¥525 million.
   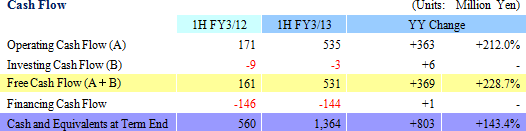 |
| Fiscal Year March 2013 Earnings Estimates |
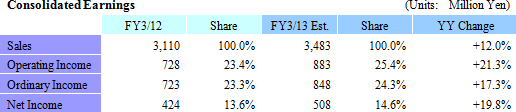 Sales, Ordinary Income Expected to Rise by 12.0%, 17.3% Year-Over-Year
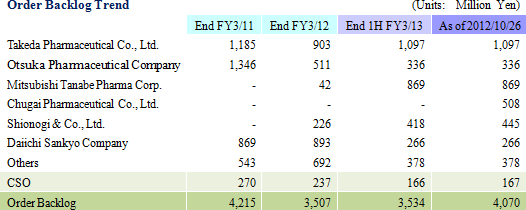
Both orders and earnings exceeded expectations during the first half. Furthermore, inquiries for projects were also stronger than expected during the first half. However, the potential for cancelation of projects could negatively impact profits due to the inability to claim project fees beyond two to three months for cancelled projects, and the subsequent potential for unutilized CRAs. Therefore, Linical states that "making an earnings revision at the current point in time with five months remaining in the fiscal year is premature." Should conditions at the end of the third quarter warrant, then the Company may decide to revise its full year earnings estimates.
|
| Conclusions |
|
Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2012 Investment Bridge Co.,Ltd. All Rights Reserved. |




