| Linical Co., Ltd. (2183) |
|
||||||||
Company |
Linical Co., Ltd. |
||
Code No. |
2183 |
||
Exchange |
First Section, TSE |
||
Industry |
Service |
||
CEO |
Kazuhiro Hatano |
||
HQ Address |
10th Fl., Shin-Osaka Brick Building, 6-1 Miyahara 1-chome, Yodogawa-ku, Osaka, Japan |
||
Year-end |
March |
||
URL |
|||
* Stock price as of closing on June 12, 2014. Number of shares issued at the end of the most recent quarter excluding treasury shares.
|
||||||||||||||||||||||||
|
|
* Estimates are those of the Company. An additional ¥2.5 per share was paid to commemorate the move of the Company's shares to the First Section of the Tokyo Stock Exchange during FY3/13.
We present this Bridge Report about Linical Co., Ltd. and details of its fiscal year March 2014 earnings. |
|
| Key Points |
 |
| Company Overview |
|
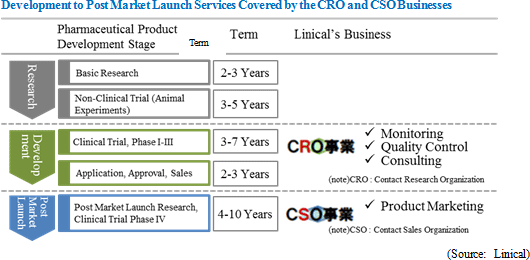
Pharmaceutical products are required to receive the approval of the Ministry of Health, Labour and Welfare prior to their sales, and efficacy and safety of pharmaceutical products must be confirmed through clinical trials prior to their approval. Companies providing clinical trial support services are known as contract research organization (CRO) service providers. The importance of CRO companies is growing as pharmaceutical companies increase their outsourcing of various services to make more effective and flexible use of their development staff and other resources, which are generally limited in number. Linical is a CRO service provider with particular strengths in the most important "phase II and III clinical trials" and know-how in the tasks of "monitoring," "quality control," and "consulting," and it seeks to maintain its position as a true partner to pharmaceutical companies by providing high quality support services. In the CRO business, Linical specializes in the most critical stages of clinical trials of phase II and III studies including major activities such as monitoring. Furthermore, Linical is able to differentiate itself from its competitors by focusing upon schizophrenia, depression, Alzheimer's disease, and other central nervous system (CNS) diseases, oncology, and other highly difficult disease realms. (Compared with these disease realms, it is more difficult to differentiate services in the realm of diseases associated with adult lifestyle habits where competition is intense.) At the same time, Linical will focus upon specific patient realms in the CSO business and leverage its know-how established in the CRO business to provide product marketing and post market launch data planning and collection tasks to differentiate its CSO business from that of its competitors, who primarily provide the service of medical representative dispatch. Linical's main customers include the Takeda Pharmaceutical Group, Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd. and other major pharmaceutical companies. Phase II clinical trials are conducted to test the safety, efficacy, usage, and dosage of pharmaceutical products. Phase III clinical trials take these results and confirm them in actual treatment conditions to test for efficacy and safety. <Corporate History>
Linical Co., Ltd. was established in June 2005 by nine members who worked at Fujisawa Pharmaceutical Co., Ltd. (Currently known as Astellas Pharma Inc.) on the development of immunosuppressant drugs. Established with the objective of becoming the ideal drug development outsourcing (CRO) company from Osaka, Linical focused its efforts in the realms of central nervous system diseases (CNS) and oncology since its founding, and received one of its first orders from Otsuka Pharmaceutical shortly after its establishment. Thereafter, the Company fortified its staffing as part of its efforts to strengthen its order taking capabilities. In addition, Linical is benefitting from its staff with bountiful experience in the realm of oncology pharmaceutical product development who formerly worked at foreign pharmaceutical companies and is seeing an expansion in orders near term.With its advance into the site management organization (SMO, clinical trial facility support organization) business, Aurora Ltd. was turned into a subsidiary in January 2006. However, all shares held in Aurora were later sold in May 2007 in order to focus management resources upon the CRO business. In July 2008, Linical USA, Inc. was established in California, United States to provide support to Japanese pharmaceutical companies seeking to enter the United States market. Also in October of the same year, Linical listed its shares on the Mothers Market of the Tokyo Stock Exchange, and subsequently moved its listing to the First Section of the Tokyo Stock Exchange in March 2013. In May 2013, Linical Taiwan Co., Ltd. and Linical Korea Co., Ltd. were established in Taiwan and Korea respectively. In April 2014, Linical teamed up with its Linical Korea to acquire the Korean CRO company P-pro. Korea Co., Ltd. 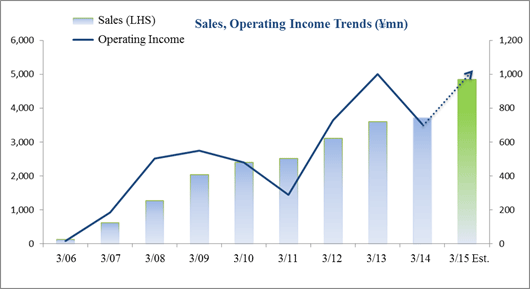 <Business Description>
Linical's business can be divided into the two main segments of the CRO and CSO businesses, with each accounting for 92.3% and 7.7% of fiscal year March 2014 sales respectively. The CRO business is focused upon "monitoring services," which includes the "quality control" and "consulting" services. At the same time, the CSO business specializes in the provision of outsourced product marketing services and post market launch data planning and collection services as a means of differentiating itself from its competitors, which primarily focus upon medical representative dispatch.
  <Strengths>
(1) Concentrated Specialized Knowledge, Know-how, Experience that Respond to Pharmaceutical Companies' Needs
In general, a long period of time of between 10 to 18 years is required to obtain approval for the commercial launch of new drugs into the market. In this overall process, clinical trials require between three to seven years to complete, and lack of preparation and data, and other problems can lead to delays in the progress of trials and in the market launch of new drugs. Linical leverages its bountiful experience in the CRA services, including its highly accurate data, speedy data gathering capability and other know-how, to predict and prevent various problems from occurring and to ensure that the "clinical trials progresses smoothly," allowing it to provide accurate explanation of the overall clinical development process at the orientation stage to clients. In particular, Linical specializes in the two most important stages of clinical trials of phase 2 and 3, and the three most important tasks of "monitoring", "quality control", and "consulting". Furthermore it is able to perform all of the consigned work internally.
(2) Strengths in CNS, Oncology Monitoring, Highly Difficult Disease Realms with Few Competitors
One of Linical's strengths is its specialization in monitoring services for highly difficult disease realms of the central nervous system and oncology, where there are few competitors. For example in the realm of oncology, evaluating symptoms to determine whether they are the result of side effects of drugs or the cancer itself is very difficult. And in the realm of central nervous system diseases, evaluating the efficacy of drugs prescribed for patients afflicted with Alzheimer's disease is also highly difficult. Therefore, high levels of responses and expertise in monitoring are necessary in these difficult realms. In addition, new drug development for patients of acute diseases and intractable diseases (so-called difficult diseases), which are also highly difficult disease realms, in addition to oncology and CNS realms is very active (There are only a few number of CROs that can respond to these situations). Evaluation of the efficacy of drugs for adult lifestyle related diseases is far easier because conditions of patients undergoing clinical trials tend to be relatively stable (For example, data gathering of blood sugar levels in diabetes patients).New drug development trends are shifting from adult lifestyle related diseases towards the realms of oncology and CNS, where treatment satisfaction is low. However as stated above, the safety evaluation of oncology drugs and the efficacy evaluation of CNS drugs are more difficult. Therefore pharmaceutical companies, which had performed these functions internally, have begun to outsource these more difficult tasks to a greater extent in recent years. The CNS realm has been a main field for Linical since its establishment, and full scale launch of order taking activities for the oncology realm was started four years ago along with the hiring of staff who developed Iressa at AstraZeneca. Order backlog in the realm of oncology is expanding. 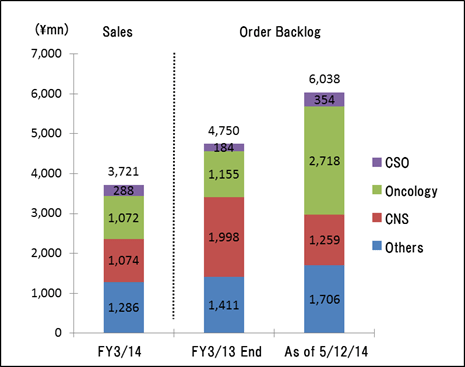 (3) High Profitability
The protocol deviation rates on projects undertaken by Linical have been held to extremely low levels, and the implementation period, including the period required for patient enrollment and data collection, for about 80% of all projects has been shortened. Because of its high levels of service quality and quick delivery times in highly difficult disease realms, Linical is able to book orders without having to offer reduced prices and is able to overcome economies of scale advantage of its larger competitors to achieve high profit margins.Moreover, the source of Linical's earnings generation capability is its highly skilled and well trained clinical research associates (CRA), as reflected in the good clinical practice (GCP) passport certification examination passage rates. The GCP passport certification examination is designed to help cultivate human resources that can respond to global joint clinical trials and is conducted by the Japan Society of Clinical Trials and Research. Moreover, all of the employees eligible to obtain qualifications have gone through the examination process, and Linical boasts of an examination pass rate far better than other companies. 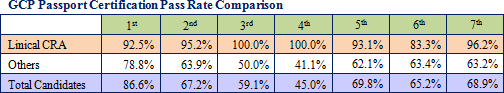  |
| Management Strategy |
|
(1) CRO Business
In the CRO business, Linical has focused upon monitoring operations in phase II and III stages of clinical trials and has received high regard from its clients for its efforts in the highly difficult disease realms of oncology and CNS. Along with further extending its track record in the realm of difficult diseases, the Company will also increase its CRA staff numbers to expand its business. Linical will endeavor to establish a structure of 270 staff working full-time as clinical research associates at an early stage.
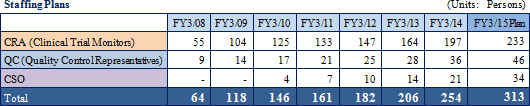 In addition, Linical is facilitating a structure to accept consignment of clinical trials in Asia (Japan, Korea, Taiwan) by hiring and cultivating local staff at companies established in respective local markets and through its global hiring and staff cultivation amidst the trend of global joint clinical trials in East Asian countries. Against this backdrop, Asian Study (Japan, Korea, Taiwan) for non-small cell cancer has been started. (2) CSO Business
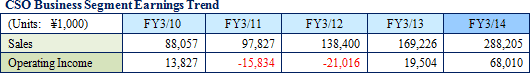
Linical differentiates itself from its competitors, which offer primarily only MR dispatch services, by focusing upon the consigned service type CSO business. Specifically, the Company seeks to differentiate itself by hiring MRs with bountiful experience in special patient realms and leveraging its bountiful know-how established in the CRO business to take on highly specialized tasks. Currently, Linical provides the two main services of product marketing and post market launch data planning and collection. Along with fortification of these two businesses in the future, the Company will also develop the global clinical research business, which is expected to expand strongly, to become the third cornerstone of this business segment. In addition, the ability to receive work for clinical research was responsible for the turn to profits of this business segment during fiscal year March 2013, and new orders for clinical research and development in fiscal year March 2014 allowed sales and profits to rise from the previous term.
  (3) Drug Discovery Support Business (New Business Development)
Linical is endeavoring to cultivate new drug discovery support services by responding to customers' needs for comprehensive "one stop shopping" services by providing new drug development schemes that have little impact upon near term earnings, aggressive development of compounds with drug lag in various Asian countries, and development plan creation and approval applications. Therefore, the Company will also focus efforts upon accumulating experiences in the strategic consignment of projects ranging from clinical trial plan creation and data collection, in addition to considering self development of compounds at an early stage by leveraging subsidies and new drug discovery funds in Japan, Korea and Taiwan. The capability to develop compounds by leveraging new drug discovery funds and experiences in this business are expected to allow Linical to expand the CRO business.
|
| Fiscal Year March 2014 Earnings Results |
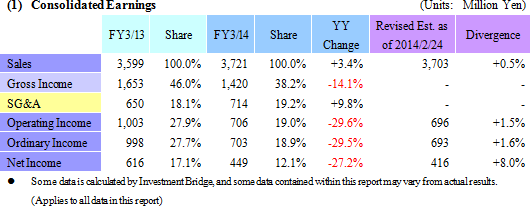 Sales Rise 3.4%, Ordinary Income Fall 29.5%
Sales rose by 3.4% year-over-year to ¥3.721 billion, but ordinary income declined by 29.5% year-over-year to ¥703 million. The CRO and CSO businesses, in which Linical participates, are undergoing gradual expansion due to the growing trend for global joint clinical trials and outsourcing of pharmaceutical product development and sales. Competition within the industry is intensifying due to restructuring of the industry arising from increases in the scale of existing participants, business transfers, and retreat from business. However order taking conditions for Linical are improving. Therefore, Linical has been successful in acquiring new consignment projects through the fortification of its marketing activities in both the CRO and CSO businesses.At the same time, the contribution of sales from new projects in the CSO business contributed positively to profits, but anticipatory investments in human resources arising from hiring and training of new staff contributed to a temporary decline in utilization rates of CRAs and caused profits of the CRO business to decline. In addition to a decline in gross income margin of 7.8% points from the previous term to 38.2%, a ¥63 million increase in sales, general and administrative expenses due to increases in commissions paid to staffing agencies caused operating income to decline by 29.6% year-over-year to ¥706 million. A slight increase was seen in non-operating income due to foreign exchange translation gains and interest received. No profits were booked at the extraordinary income level. A yearend dividend of ¥14 per share is expected to be paid. 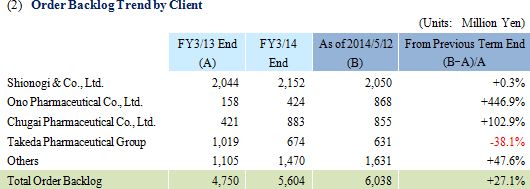 As of May 12, 2014, order backlog were 27.1% higher than backlog at the end of the fiscal year March 2013. This result is a reflection of the favorable order environment arising from the growing trend for global joint clinical trials and outsourcing of various services, and is better than indicated by the current level of sales. The large number of consignment project inquiries from existing and new clients due to the favorable near term order environment and the success of marketing activities is the reason for steps taken to fortify its consignment structure through the increase in CRAs.
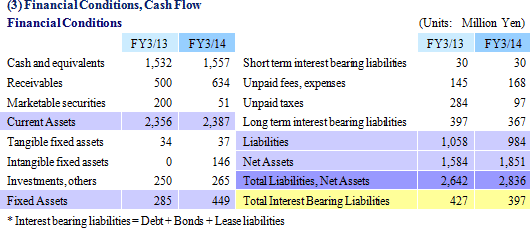 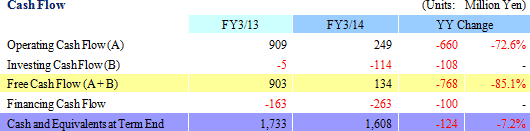 |
| Fiscal Year March 2015 Earnings Estimates |
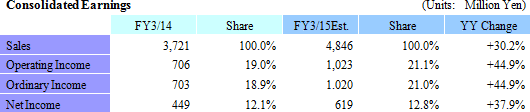 Sales, Ordinary Income Expected to Rise 30.2%, 44.9% in FY3/15
Linical's fiscal year March 2015 earnings estimates call for sales and ordinary income to rise by 30.2% and 44.9% year-over-year to ¥4.846 and ¥1.020 billion respectively. With regards to sales, the CRO business has been able to maintain high regard from clients since the Company's founding in addition to receiving repeat orders from existing customers. At the same time, the CRO business is expected to cultivate orders for new projects in the realms of oncology and central nervous system ailments where needs of customers is strong. In the CSO business, marketing efforts have been fortified to cultivate business from new customers and know-how developed in the CRO business is expected to be leveraged to cultivate orders for new projects in highly specialized realms. With regards to profits, cost of sales and sales, general and administrative expenses are expected to increase due to global development and an increase in CRA (clinical development monitor) staffing. However the positive impact of higher sales and higher capacity utilization rates of CRAs are expected to allow profits to rise by a large margin. A dividend of ¥14 per share is expected to be paid at the term end.
|
| Conclusions |
|
Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2014 Investment Bridge Co.,Ltd. All Rights Reserved. |




