| Linical Co., Ltd. (2183) |
|
||||||||
Company |
Linical Co., Ltd. |
||
Code No. |
2183 |
||
Exchange |
First Section, TSE |
||
Industry |
Service |
||
CEO |
Kazuhiro Hatano |
||
HQ Address |
10 Fl., Shin-Osaka Brick Building, 6-1 Miyahara 1-chome, Yodogawa-ku, Osaka, Japan |
||
Year-end |
March |
||
URL |
|||
* Stock price as of closing on May 24, 2016. Number of shares issued at the end of the most recent quarter excluding treasury shares.
|
||||||||||||||||||||||||
|
|
* Estimates are those of the Company. An additional dividend of ¥2.5 per share was paid to commemorate the move of the Company's shares to the First Section of the Tokyo Stock Exchange during FY3/13
* A 2 for 1 stock split was performed on January 1, 2016. Dividend payment after the stock split is ¥10 per share (Including a ¥1 commemorative dividend).The actual dividend would be ¥20 per share if the stock split had not been conducted and would represent a ¥6 per share increase from the previous term. We present this Bridge Report about Linical Co., Ltd. and details of fiscal year March 2016 earnings. |
| Key Points |
 |
| Company Overview |
|
Linical has conducted various efforts to eradicate cancer, central nervous system and other diseases globally since its founding, and it has deployed its CRO business in disease realms where there is strong demand for new drug development. These are highly difficult disease realms, and Linical is able to support clinical trials in these realms with its high levels of knowledge and bountiful expertise. In addition, Linical focuses its efforts up the new drug development support and contract medical affairs business, approval application support and post approval marketing and clinical research, and post market survey support services, which exceed the traditional definition of outsourcing and is now considered to be part of a wider range of consulting services provided to customers as a "true clinical development partner". Furthermore, amidst the advance of globalization and large scale pharmaceutical product development, the Linical Group can provide "one stop shopping" type comprehensive services for large scale global products. Consequently, Linical is able to play the role of a strategic business partner by providing total support to help raise the competitive advantage of customers in the market and to help pharmaceutical companies develop new future business opportunities. 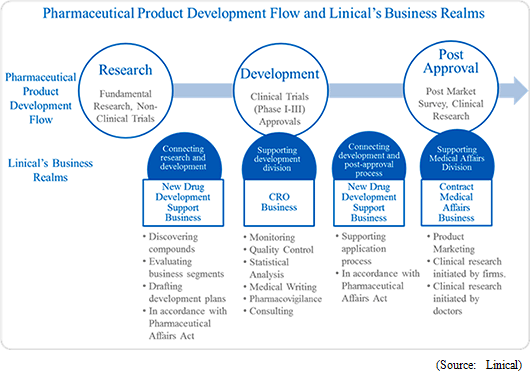 <Corporate History>
Linical Co., Ltd. was established in June 2005 by nine members who worked at Fujisawa Pharmaceutical Co., Ltd. (Currently known as Astellas Pharma Inc.) on the development of immunosuppressant drugs. Established with the objective of becoming the ideal drug development outsourcing (CRO) company from Osaka, Linical focused its efforts upon the realms of central nervous system diseases (CNS) and oncology since its founding, and received one of its first orders from Otsuka Pharmaceutical Company shortly after its establishment. Thereafter, the Company fortified its staffing as part of its efforts to strengthen its order taking capabilities. In addition, Linical is benefitting from the bountiful experiences of its employees in the realm of oncology pharmaceutical product development and experiences having worked at foreign pharmaceutical companies. Consequently, Linical is successfully expanding orders in the near term.With its advance into the site management organization (SMO, clinical trial facility support organization) business, Aurora Ltd. was turned into a subsidiary in January 2006. However, all shares held in Aurora were later sold in May 2007 in order to focus management resources upon the CRO business. In July 2008, Linical USA, Inc. was established in California, United States to provide support to Japanese pharmaceutical companies seeking to enter the United States market. Also in October of the same year, Linical listed its shares on the Mothers Market of the Tokyo Stock Exchange, and subsequently moved its listing to the First Section of the Tokyo Stock Exchange in March 2013. In May 2013, Linical Taiwan Co., Ltd. and Linical Korea Co., Ltd. were established in Taiwan and Korea respectively. In April 2014, Linical teamed up with its Linical Korea to acquire the Korean CRO company P-pro. Korea Co., Ltd. In October 29, 2014, all of the shares of Nuvisan CDD Holding GmbH, which conducts CRO business in Europe, were acquired and it was converted to a 100% owned subsidiary effective on December 1, 2014. In order to strengthen the collaboration within the Group, the company name of Nuvisan CDD was changed to Linical Europe GmbH. 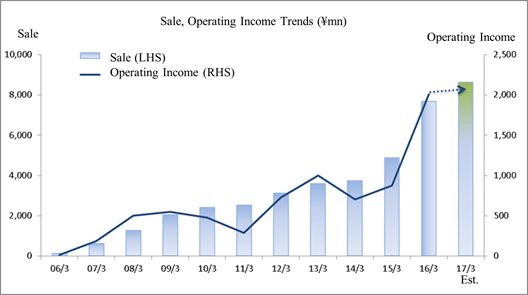 <Business Description>
CRO Business
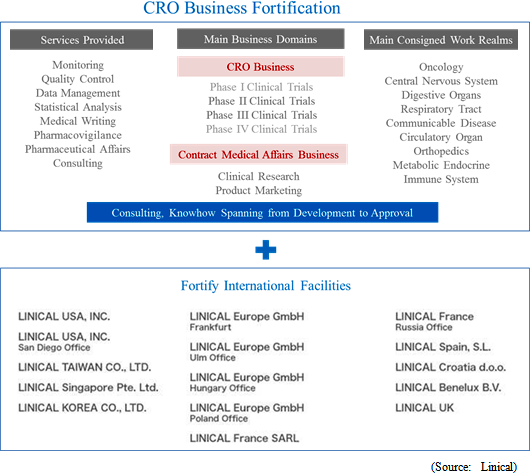
In the main CRO business, Linical seeks to provide highly effective clinical trial support to allow the quick introduction of new drugs into the market by maintaining staff with high levels of technological knowledge and bountiful experiences. The Company opened facilities in Asia (Korea, Taiwan and Singapore), Europe and the United States to be able to respond to growing demand for global studies. Llinical provides "one stop shopping" services ranging from pharmaceutical affairs to planning, implementation plan creation, monitoring, data management, statistical analysis, and pharmacovigilance. With regards to jointly conducted global trials, Linical headquarters operates a function where personnel with in depth knowledge about various countries pharmaceutical product development work. These personnel are able to provide information necessary to establish a development environment that can enable jointly conducted global clinical trials to be conducted in Japanese. Within new drug development projects, which take between 10 to 20 years, the Company specializes in the clinical trial process where patients are subjected to treatments in the "phase II" and "phase III" stages of clinical trials, which require between 3 to 7 years, and monitoring, quality management, and consulting services, which make up the core of clinical trials, are provided. Highly credible data is accumulated in support services to ensure quick and steadfast new drug development can be conducted. Moreover, Linical has chosen to specialize in oncology and central nervous system related realms, where development demands from the market are strong, and other highly difficult disease realms and to respond to the needs of pharmaceutical companies in these realms.
 Contract Medical Affairs Business
Clinical research after pharmaceutical product approval entails evidence based medicine (EBM) data creation related to activities designed to improve the quality of medical treatment including validation of effectiveness, confirmation of safety, and interaction consideration for pharmaceutical product usage in the clinical work front. In recent years, facilitation of rules for clinical research has progressed, and the amount of corporate led clinical research is on the rise, in addition to the traditional clinical research. Linical has been able to implement clinical research that responds to the most recent information in global applications not only in intervention studies, but also in consigned observational studies, database research and other various clinical research by leveraging its knowhow in the realm of clinical trials. In the future, pharmaceutical companies will gather opinions from key opinion leaders (KOL) in various disease realms through its medical affairs division (MA) and medical science liaison (MSL) along with the increasing fairness and transparency of marketing activities. Various tasks are conducted within the MSL division including key opinion leader engagement, advice board operations, and lecture operations. Linical will leverage its experiences in creation of operating manuals, regional opinion leader support, clinical research procedure manual creation support and consulting services to provide medical science liaison support on a global basis.
 New Drug Development Support Business
Linical provides a wide range of services that respond to the needs of pharmaceutical product development tasks including consigned clinical trial monitoring, new drug marketing support, development plan proposal, and clinical trial plan creation that fully satisfy the requirements of client pharmaceutical companies in the new drug development support business. Moreover, Linical proposes new drug development solutions that reduce the risk of development by leveraging funds and subsidies to develop compounds that become the "seeds of medicine" in the initial stages of pharmaceutical product development process.
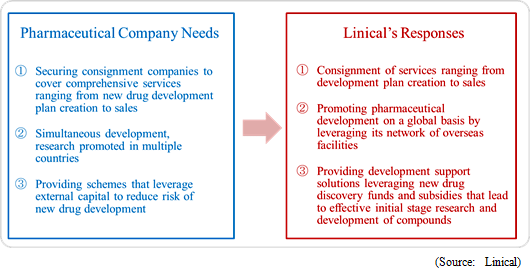 <Strengths>
(1) Concentrated Specialized Knowledge, Knowhow, Experience that Respond to Pharmaceutical Companies' Needs
In general, a long period of time, between 10 to 18 years, is required to obtain approval for the commercial launch of new drugs into the market. In this overall process, clinical trials require between three to seven years to complete, and lack of preparation, data, and other issues can lead to delays in the progress of trials and in the market launch of new drugs. Linical leverages its bountiful experience in the CRA services, including its highly accurate and speedy data gathering capability, other knowhow to predict and prevent various problems from occurring and ability to ensure that the "clinical trials progress smoothly," allowing it to provide accurate explanations of the overall clinical development process at the orientation stage to clients. Furthermore, a characteristic of Linical's specialized CRO service is its focus upon the three highly efficient tasks mentioned below.
① Linical specializes in the three core tasks ("monitoring", "quality control" and "consulting") of development, and it maintains a structure that is able to perform 100% of this consigned work internally.
② The Company focuses upon the two most important stages of clinical trials of phase II and III.
③ Linical focuses upon major pharmaceutical companies that possess bountiful pharmaceutical product development information.
(2) Strengths in CNS, Oncology Monitoring, Highly Difficult Realms with Few Competitors
One of Linical's strengths is its specialization in monitoring services for highly difficult disease realms of the central nervous system (CNS) and oncology, where there are few competitors. For example, evaluating symptoms to determine whether they are the result of side effects of drugs or the cancer itself in the realm of oncology is very difficult. And in the realm of central nervous system diseases, evaluating the efficacy of drugs prescribed for patients afflicted with Alzheimer's disease is also highly difficult. Therefore, sophisticated responses and expertise in monitoring are necessary in these difficult realms. In addition, new drug development for patients of acute and intractable diseases (Difficult diseases), which are also highly difficult disease realms, oncology and CNS disease realms is very active (There are only a few CROs that can respond to these situations). An evaluation of the efficacy of drugs for adult lifestyle related diseases is far easier because conditions of patients undergoing clinical trials tend to be relatively stable (Data gathering of blood sugar levels in diabetes patients).New drug development trends are shifting from adult lifestyle related diseases towards the realms of oncology and CNS, where treatment satisfaction is low. However as stated above, the safety evaluation of oncology drugs and the efficacy evaluation of CNS drugs are more difficult. Therefore, there is a trend of aggregation of CROs with bountiful experiences in consigned tasks in these realms. Order backlog in the realm of oncology, CNS, acute diseases and intractable diseases are expanding. 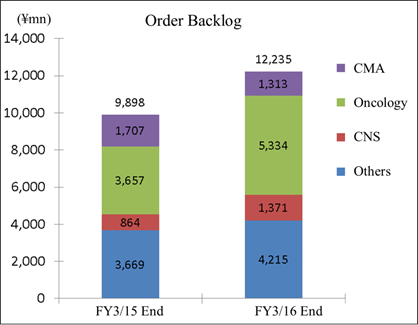  (3) High Profitability
The protocol deviation rates on projects undertaken by Linical have been held to extremely low levels, and the implementation period, including the period required for patient enrollment and data collection, for about 80% of all projects has been shortened. Because of its high levels of service quality and quick delivery times in highly difficult disease realms, Linical is able to receive orders without having to offer reduced prices, and it is able to achieve high profit margins by overcoming the economies of scale advantage of its larger competitors.Moreover, the source of Linical's earnings generation capability are its highly skilled and well trained clinical research associates (CRA), as reflected in the good clinical practice (GCP) passport certification examination passage rates. The GCP passport certification examination is designed to promote improvements in the quality of Japan's clinical trials and clinical research and is conducted by the Japan Society of Clinical Trials and Research. Moreover, all of the employees eligible to obtain qualifications have gone through the examination process, and Linical achieved passage rates which are much higher than its competitors. 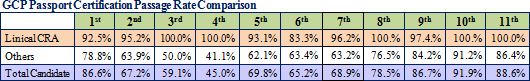 |
| Management Strategy |
|
(1) CRO Business
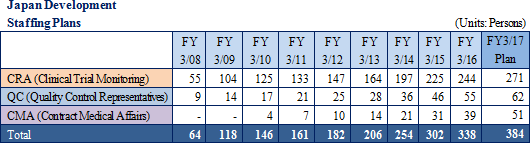
The main business strategy within the CRO business calls for 1) continued results to be achieved in the realms of oncology and central nervous systems, with repeat orders being converted into exclusive contracts, 2) early achievement of a structure with 300 CRAs within Japan while developing the volume in US and Taiwan and 3) implementation of a global structure that enables one stop consignment order taking capability for jointly conducted global clinical trials.
 Global Development
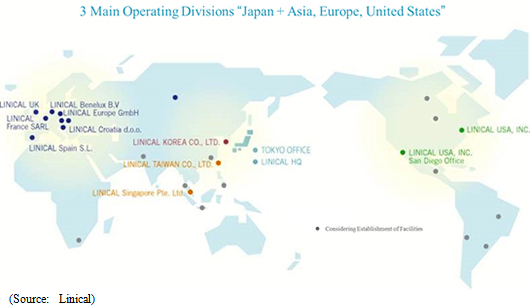
Linical will leverage its global consignment business structure for jointly conducted global clinical trials conducted in Asia, North America and Europe to accelerate the expansion in its global business. As part of the facilitation of a structure for clinical trials conducted between multiple countries, Linical Taiwan Co., Ltd. (Taipei City, Taiwan, capitalized at Taiwan $10 million) and Linical Korea Co., Ltd. (Seould City, Korea, capitalized at 1 billion won) have been established as 100% owned subsidiaries. In April 2014, Linical Korea Co., Ltd. was merged with the Korean CRO service provider P-pro. Korea Co., Ltd. And in July 2008, Linical USA, Inc. (New Jersey, USA) was established, and an office was opened in San Diego, California in September 2014 to expand business in the United States. Furthermore, Linical reached an agreement with Nuvisan Pharma Holding GmbH to acquire Nuvisan CDD Holding GmbH as a subsidiary to provide CRO services in Europe on October 29, 2014, with completion of the acquisition on December 1, 2014. At the same time, the company name was changed to Linical Europe GmbH. These developments have created a consignment business structure in the three regions of Asia, North America and Europe.
  (2) Contract Medical Affairs Business
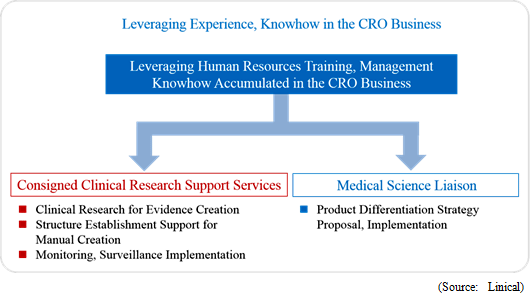
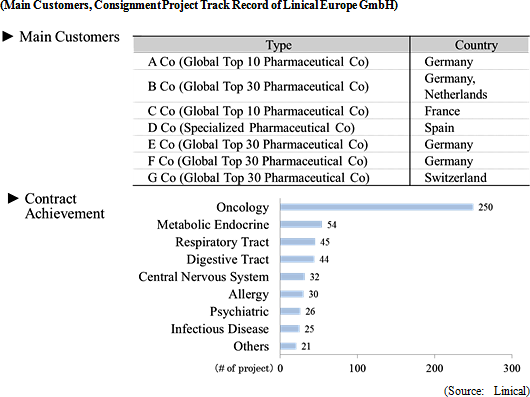
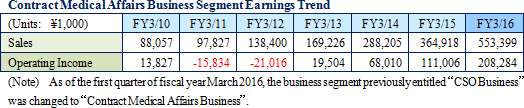
Linical differentiates itself from its competitors, which primarily offer only MR dispatch services, by focusing upon the consigned service type drug development business. Specifically, the Company seeks to differentiate itself by hiring MRs with bountiful experience in special patient realms and leveraging its bountiful knowhow established in the CRO business to take on highly specialized tasks. Currently, Linical provides the two main services of clinical research support and product marketing (liaison). With regards to the provision of consigned support services for clinical research, the securing of quality levels of clinical research is a critical issue for evidence creation. Linical creates procedure manuals for structure facilitation support, monitoring and surveillance implementation. Cultivation of new medical institutions and physicians as clients in the process for market launch of new products in new realms, and execution of product differentiation strategies are conducted and proposed in the product marketing (liaison) service. In addition, the ability to receive work for clinical research was responsible for the turn to profits of this business segment during fiscal year March 2013, and new orders for clinical research and development in fiscal year March 2014 allowed sales and profits to rise from the previous term.
 (3) New Drug Development Support Business (New Business Development)
Linical is endeavoring to cultivate new drug development support services by responding to customers' needs for comprehensive "one stop shopping" services by providing new drug development schemes that have little impact upon near term earnings and for which demand is growing. In addition, patients and the administrative authorities in Taiwan and Japan are encouraging companies to develop drug lag compounds as well as new drugs in the realms of oncology, dementia, difficult diseases and regenerative medical treatments. Linical seeks to cultivate a new drug development support business that responds to these needs which have surfaced in recent years. Therefore, the Company will implement efforts for clinical trial implementation planning creation, data collection and other tasks, and plan development of compounds in house at an early stage by leveraging subsidies and new drug development funds. Development of compounds using new drug funds is expected to help grow the CRO business while allowing the experiences of the Company to also be leveraged. Also, experiences in consignment of tasks ranging from development planning to approval applications are expected to be gathered.
|
| Fiscal Year March 2016 Earnings Results |
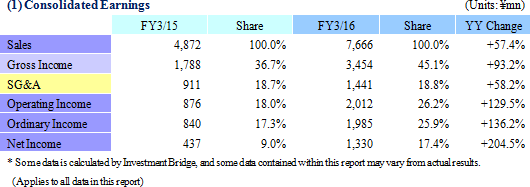 Sales, Ordinary Income Rise by 57.4%, 136.2%
Sales and ordinary income rose by 57.4% and 136.2% year-on-year to ¥7.666 and ¥1.985 billion respectively. Against the backdrop of increases in outsourcing of pharmaceutical product development and sales, and jointly conducted global clinical trials, the market scale of the CRO and CSO industries, to which Linical belongs, is gradually growing. Amidst these trends, aggressive efforts to create a global consignment structure covering Japan, Asia, the United States and Europe and subsequent favorable orders trends in Japan and overseas markets, and for jointly conducted global trials allowed the CRO business to expand. The contract medical affairs also saw an increase in post market launch clinical research consignment projects. With regards to profits, favorable earnings of the CRO business within Japan and a large contraction in losses and return to profitability of overseas subsidiaries contributed to the strong increase in profits. In addition, increases in consigned projects within the contract medical affairs business contributed to an increase in the utilization rates of staff and an increase in profits. Increases in staffing within Japan and fortification of operations in Europe through M&A contributed to increased labor costs, but gross income margin still managed to rise by 8.4% points year-on-year to 45.1%. At the same time, amortization for goodwill resulting from M&A activities in Europe and fortification of activities caused sales, general and administrative expenses to rise and SG&A margin rose by 0.1% point year-on-year to 18.8%. As a result of these developments, operating income rose by 129.5% year-on-year to ¥2.012 billion. In addition, foreign exchange translation loss declined by ¥20 million year-on-year within non-operating income and allowed ordinary income margin to increase. At the same time, the disappearance of ¥105 million in retirement benefit expenses booked in the previous term also allowed parent net income to sales margin to rise. By geographic region, the influence of capital investments arising from an increase in staffing in the United States was offset by better than expected performances in Europe, Taiwan, Korea and other regions.A two for one stock split was implemented on January 1, 2016. After the stock split, the dividend payment was adjusted to ¥10 per share (Including a ¥1 commemorative dividend). The actual dividend would be ¥20 per share if the stock split had not been conducted and it would represent a ¥6 per share increase from the previous term. 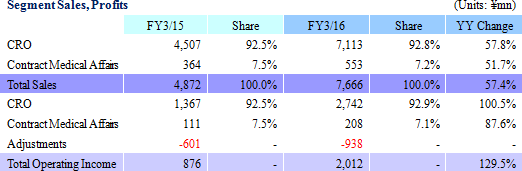 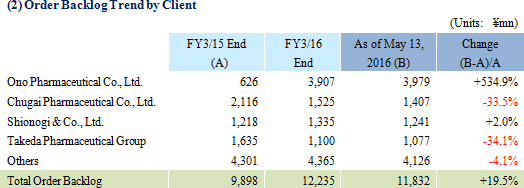 As of May 13, 2016, order backlog were 19.5% higher than the level recorded at the end of fiscal year March 2015. This favorable figure also reflects the steady booking of backlog as sales which were exceeded by a stronger inflow of new orders. Linical has plans to strengthen its consignment structure by hiring additional CRA (Clinical development monitoring) staff in response to the favorable near term order environment with increases in outsourcing and jointly conducted global clinical trials, and large number of inquiries from both new and existing clients for consigned projects. 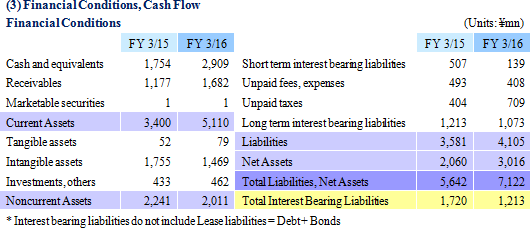 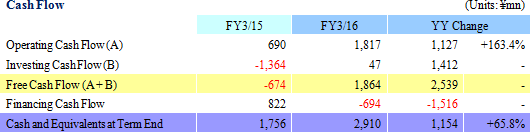 |
| Fiscal Year March 2017 Earnings Estimates |
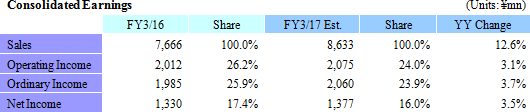 Sales, Ordinary Income Expected to Rise by 12.6%, 3.7%
According to Linical's earnings estimates for fiscal year March 2017, sales and ordinary income are expected to rise by 12.6% and 3.7% year-on-year to ¥8.633 and ¥2.060 billion respectively. In order to offset the negative influence of major products going off patent, major pharmaceutical companies are increasing their outsourcing as a means of rationalizing and optimizing their management, as well as focusing their efforts on discovering highly promising pharmaceutical product categories. At the same time, acquisition of overseas venture companies is accelerating, and the number of consigned clinical trials for new drug development is anticipated to increase. In particular, there is strong need for development of treatments in the realms of oncology and central nervous system diseases for which there are still no effective treatments, and new drug development responding to these needs is on the rise. Against this backdrop, Linical will endeavor to acquire repeat businesses from existing customers who regard Linical's services highly and to cultivate business with new clients through strengthened marketing activities. In particular, Linical boasts of strengths in oncology and central nervous system related disease realms for which customers' needs are high, and it will pursue expansion of sales through the acquisition of consigned work projects for jointly conducted global clinical trials. With regards to profits, the Company will endeavor to maintain high CRA capacity utilization rates to grow profits despite the negative influence of amortization of goodwill arising from acquisition of Korean and European subsidiaries is expected to continue, along with capital investments for overseas subsidiaries. Furthermore, strengthening of marketing activities to new customers will be conducted in the contract medical affairs business, and efforts to acquire new consignment projects in realms of high specialization by leveraging knowhow accumulated in the CRO business will be conducted. Operating income is expected to rise by 3.1% year-on-year to ¥2.075 billion. Increases in staff numbers within Japan and in capital investments arising from an expansion of operations in the United States have been factored into these estimates, and are expected to contribute to the conservative outlook for a 2.2% point year-on-year decline in operating income margin to 24%. In addition, there are no expectations for significant bookings profits or expenses at the non-operating or extraordinary income levels. While Linical's estimates for fiscal year March 2017 are based on the sum of the plans for its various operating regions, they do not reflect any synergies including potential orders for jointly conducted global clinical trials derived from strengthening of the global structure and overseas facilities. |
| Conclusions |
|
In addition, Linical's estimates for 12.6% and 3.7% year-on-year growth in sales and ordinary income during fiscal year March 2017 appear to be very conservative. This estimate for sales is derived from the sum of its sales estimates in each geographic operating region, and they do not reflect any synergies including potential orders for jointly conducted global clinical trials derived from strengthening of the global structure and overseas facilities. Moreover, profit estimates are based on the assumption of aggressive capital investments for facilities in Japan, the United States and Taiwan. Order values for comprehensive jointly conducted global clinical trials are expected to be significantly larger than traditional orders booked only in single operating regions. Consequently, the contributions to profits which can be derived from the synergy effect of these large jointly conducted global clinical trials are expected to be large. Therefore, the market is also likely to keep a close watch upon Linical's order trends and its ability to capture these large jointly conducted global trials. |
| <Reference: Corporate Governance> |
 ◎ Corporate Governance Report
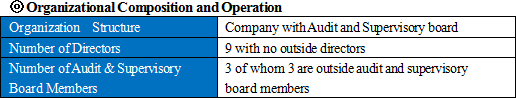
Linical Co., Ltd. has submitted a Corporate Governance Report on June 24, 2016 after the Corporate Governance Code came into effect.<Basic concept> The Company contributes to the development of pharmaceutical products through its technologies as a partner to major pharmaceutical companies in Japan and responds to the expectations from the entire society in the field of pharmaceutical products. Furthermore, the Company believes that it is necessary to establish a system that enables prompt decision making with soundness and transparency to enhance its corporate value. Thus, the Company is making efforts to strengthen the internal control system including strict compliance, which is currently the Company's highest priority. <Implementation of corporate governance codes and principles> Reasons for Non-compliance with the Principles of the Corporate Governance Code (15 items)   The Company has not elected any outside director yet. CGC encourages companies to have multiple outside directors, and more than 90% of the listed companies have outside directors (June 2015, Tokyo Stock Exchange). Since the amendment of Companies Act last year, an increasing number of companies have been shifting to the company with audit and supervisory board, but many companies meet the requirement of multiple outside directors by simply changing the existing outside auditors to directors. The Company can also institutionally fulfill the requirement because it already has 3 outside auditors. However, the Company purposefully does not take the option. Instead, it examines the appropriateness for the Company to carry out the corporate governance codes/principles based on its unique situation and provides specific reasons why they do not carry it out at the moment. The Company is planning to examine the options for the method to exercise voting rights at the shareholders' meetings and possibility of disclosures in case there are many negative votes. Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2016 Investment Bridge Co., Ltd. All Rights Reserved. |




