Bridge Report:(2183)Linical the first half of the fiscal year March 2020
 Kazuhiro Hatano CEO | Linical Co., Ltd. (2183) |
 |
Company Information
Market | TSE 1st Section |
Industry | Service |
CEO | Kazuhiro Hatano |
HQ Address | 10 Fl., Shin-Osaka Brick Building, 6-1 Miyahara 1-chome, Yodogawa-ku, Osaka, Japan |
Year-end | March |
HP |
Stock Information
Share Price | Number of shares issued (excluding treasury shares) | Total market cap | ROE Act. | Trading Unit | |
¥1,158 | 22,586,555 shares | ¥26,155 million | 10.9% | 100 shares | |
DPS Est. | Dividend yield Est. | EPS Est. | PER Est. | BPS Act. | PBR Act. |
¥14.00 | 1.21% | ¥43.02 | 26.9x | ¥224.48 | 5.2x |
* Stock price as of closing on December 17, 2019. Number of shares issued at the end of the most recent quarter excluding treasury shares.
* ROE is based on previous term’s results. EPS and DPS are based on the estimates of FY3/20.
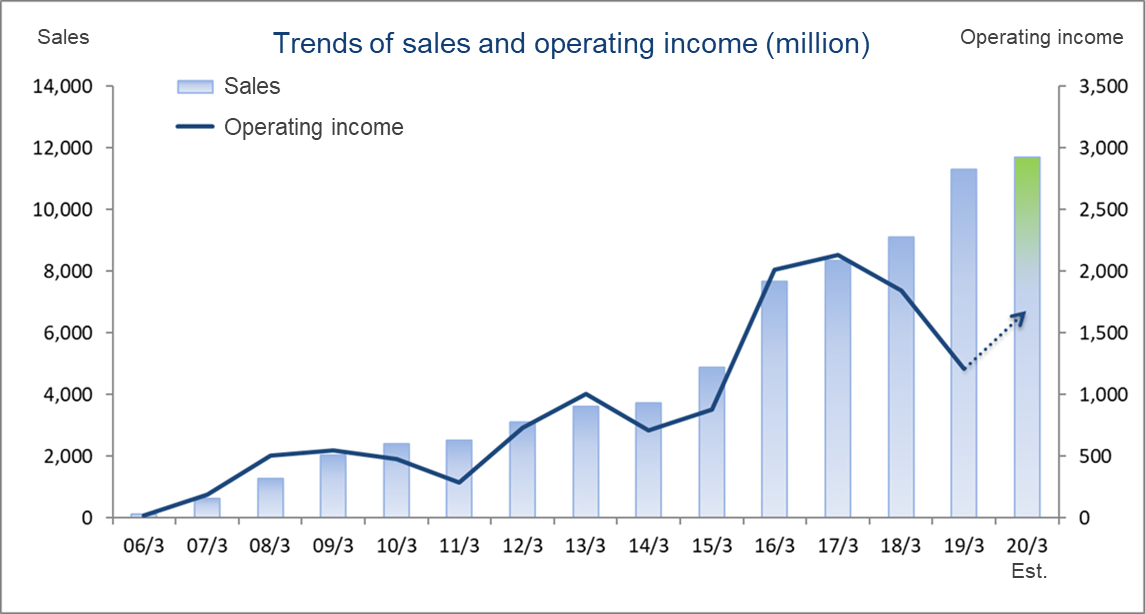
Consolidated Earnings Trend
Fiscal Year | Sales | Operating Income | Ordinary Income | Parent Net Income | EPS | DPS |
March 2016 Act. | 7,666 | 2,012 | 1,985 | 1,330 | 58.40 | 10.00 |
March 2017 Act. | 8,355 | 2,128 | 2,076 | 1,447 | 63.59 | 10.00 |
March 2018 Act. | 9,113 | 1,846 | 1,826 | 1,295 | 57.02 | 11.00 |
March 2019 Act. | 11,313 | 1,212 | 1,253 | 568 | 25.09 | 12.00 |
March 2020 Est. | 11,700 | 1,560 | 1,538 | 971 | 43.02 | 14.00 |
* Estimates are those of the Company. ¥1 per share was paid during FY3/16 to commemorate the 10th anniversary of its establishment.
*A 2 for 1 stock split was performed on January 1, 2016. Dividend payment after the stock split is ¥10 per share (Including a ¥1 commemorative dividend), the actual dividend payment would have been ¥20 per share if the stock split is not considered.
We present this Bridge Report about Linical Co., Ltd. and details of the first half of the fiscal year March 2020 earnings.
Table of Contents
Key Points
1. Company Overview
2. Management Strategy
3. The First Half of Fiscal Year March 2020 Earnings Results
4. Fiscal Year ending March 2020 Earnings Forecasts
5. Conclusions
<Reference:Regarding Corporate Governance>
Key Points
- For the first half of fiscal year March 2020, sales and ordinary income declined 4.0% and 35.2%, respectively, year on year. As for sales, the yen appreciation decreased the sales of overseas subsidiaries in Japanese yen, and several large-scale projects ended in Japan from the previous term to the first half of this term and new projects are still to be started. These factors affected sales. Profit was in line with the initial estimate as a whole, but declined because sales decreased year on year and the company paid lawyers’ remunerations for negotiating about the closing price of a subsidiary in the U.S. with the seller.
- Linical’s fiscal year March 2020 earnings estimates, which were not revised, call for sales and ordinary income to rise by 3.4% and 22.8% year-on-year respectively. With regard to sales, through repeat orders from existing customers and cultivation of orders from new customers, it aims to accept new projects including global jointly conducted clinical trials, focusing on the oncology and central nervous system (CNS) disease realms at which the Group excels. With regard to profits, although goodwill amortization associated with M&A of European and American subsidiaries will increase, Linical will strive to increase global jointly conducted clinical trials by strengthening the management base of overseas subsidiaries and expanding the scale of the North American business in order to improve profitability. Dividend is still expected to be ¥14 per share, up ¥2 per share from the previous fiscal year (a regular dividend of ¥13 per share, an increase of ¥1 per share from the previous fiscal year, in addition to the commemorative dividend of ¥1 per share to commemorate the consolidated net sales exceeding 10 billion yen in the fiscal year March 2019).
- Since the start of business, the company has conducted the CRO business, specializing in cancer, the central nervous system, and the immune system. The company will continue the business while focusing on these three fields, but plans to enter the fields of regenerative medicine, dermatology, and ophthalmology, where the number of clinical trials is expected to increase considerably. Their growth strategies in the promising fields of regenerative medicine, dermatology, and ophthalmology are noteworthy.
1.Company Overview
Linical Co., Ltd. provides contract research organization (CRO) services that support the drug development processes of pharmaceutical companies on an outsourced consignment basis, and sales and marketing functions for pharmaceutical products and post market launch clinical research and surveys on a consigned basis in the contract medical affairs business (CMA). Pharmaceutical products are subject to approval of the Ministry of Health and Welfare prior to their sales, and efficacy and safety of pharmaceutical products must be confirmed through clinical trials prior to their approval. Companies providing clinical trial support services are known as contract research organization (CRO) service providers. In addition, there is a need to conduct surveys and clinical research after pharmaceutical products have been launched into the market and contract medical affairs is a service provided to support these efforts.
Since its founding, Linical has deployed its CRO business in disease realms where there is strong demand for new drug development and people wish for the eradication of cancer, central nervous system and other diseases globally. These are highly difficult disease realms, and Linical is able to support clinical trials in these realms with its high levels of knowledge and bountiful expertise. In addition, Linical focuses its efforts up the new drug development support and contract medical affairs business, approval application support and post approval marketing and clinical research, and post market survey support services, which exceed the traditional definition of outsourcing and is now considered to be part of a wider range of consulting services provided to customers as a "true clinical development partner". Furthermore, amidst the advance of globalization and large scale pharmaceutical product development, the Linical Group can provide "one stop shopping" type comprehensive services for large scale global products. Consequently, Linical is able to play the role of a strategic business partner by providing total support to help raise the competitive advantage of customers in the market and to help pharmaceutical companies develop new future business opportunities.
Furthermore, Linical has a contract-based business style and is establishing a highly profitable structure. It is focusing on specific businesses (i.e. monitoring, quality control, and consulting, which are the main activities of clinical trials), specific clinical trials (i.e. Phase II and Phase III) and specific customers (i.e. major pharmaceutical companies with abundant medical product development information).
【1-1 Corporate History】
Linical Co., Ltd. was established in June 2005 by nine members who worked at Fujisawa Pharmaceutical Co., Ltd. (Currently known as Astellas Pharma Inc.) on the development of immunosuppressant drugs. Established with the objective of becoming the ideal drug development outsourcing (CRO) company from Osaka, Linical focused its efforts upon the realms of central nervous system diseases (CNS) and oncology since its founding, and received one of its first orders from Otsuka Pharmaceutical Company shortly after its establishment. Thereafter, the Company fortified its staffing as part of its efforts to strengthen its order taking capabilities. In addition, Linical is benefitting from the bountiful experiences of its employees in the realm of oncology pharmaceutical product development and experiences having worked at foreign pharmaceutical companies. Consequently, Linical is successfully expanding orders in the near term.
With its advance into the site management organization (SMO, clinical trial facility support organization) business, Aurora Ltd. was turned into a subsidiary in January 2006. However, all shares held in Aurora were later sold in May 2007 in order to focus management resources upon the CRO business. In July 2008, Linical USA, Inc. was established in California, United States to provide support to Japanese pharmaceutical companies seeking to enter the United States market. Also, in October of the same year, Linical listed its shares on the Mothers Market of the Tokyo Stock Exchange, and subsequently moved its listing to the First Section of the Tokyo Stock Exchange in March 2013. In May 2013, Linical Taiwan Co., Ltd. and Linical Korea Co., Ltd. were established in Taiwan and Korea respectively. In April 2014, Linical teamed up with its Linical Korea to acquire the Korean CRO company P-pro. Korea Co., Ltd. In October 29, 2014, all of the shares of Nuvisan CDD Holding GmbH, which conducts CRO business in Europe, were acquired and it was converted to a 100% owned subsidiary effective on December 1, 2014. In order to strengthen the collaboration within the Group, the company name of Nuvisan CDD was changed to Linical Europe GmbH. In addition, Linical U.K. Ltd. was established in March 2016, and a local subsidiary called Linical Poland SP. Z.O.O. was also established in October of the same year. Moreover, LINICAL Czech Republic s.r.o was established in September 2017. In addition, Accelovance, Inc. was acquired in April 2018 and its company name was changed to Linical Accelovance America, Inc. This acquisition has contributed to a strengthening of Linical's consignment structure for global jointly conducted clinical trials.
Furthermore, LINICAL Hungary Kft. was established in March 2019, and LINICAL CHINA Co., Ltd. was established in May 2019. As a result, the Group now has a stronger system to receive contracts for global jointly conducted clinical trials.
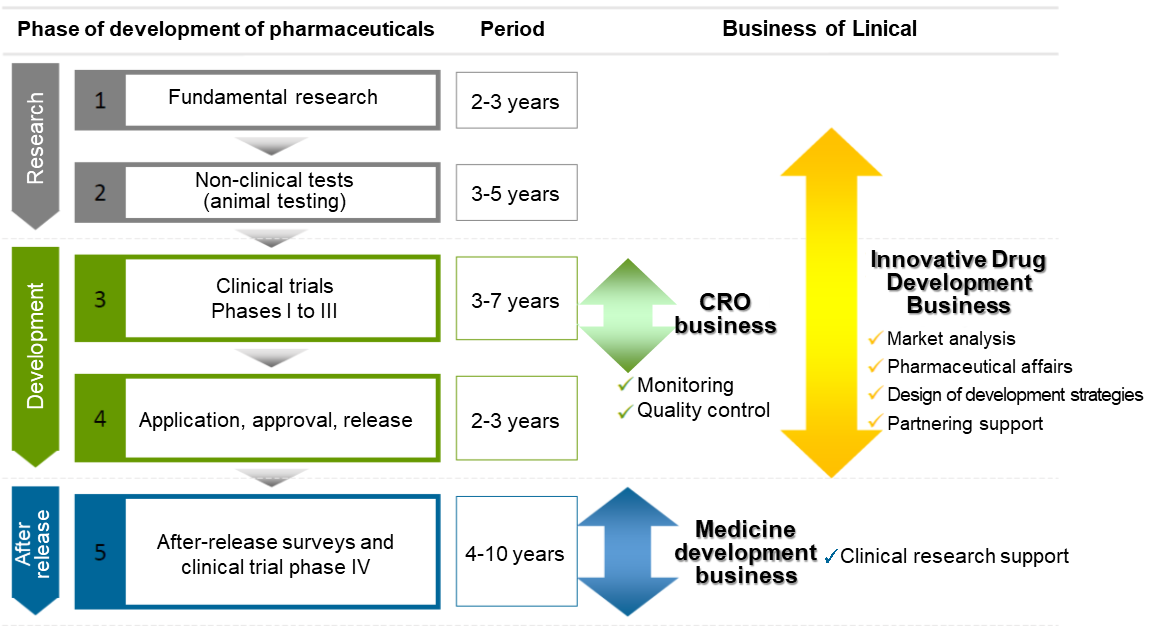
【1-2 Business Description】
Linical mainly conducts contract research organization (CRO) business, post market launches clinical trial and clinical research and marketing support activities in the contract medical affairs business, and new drug development support business.
As a true partner, the company contributes to the maximization of the value of the medical drugs by helping the procedure from the non-clinical tests to clinical development and after-release surveys and clinical trial, and making it possible to shorten the time needed to start selling the drugs and prolong the life-cycle of the products. On top of that, the company supports not only pharmaceutical companies but also the bio-ventures in various ways including exit strategies.
(Source Linical)
CRO Business (Contract Research Organization)
The mainstay CRO Business is characterized by business-specific CROs. In the main CRO business, Linical seeks to provide highly effective clinical trial support to allow the quick introduction of new drugs into the market by maintaining staff with high levels of technological knowledge and bountiful experiences. The company has opened facilities in Asia (Korea, Taiwan, Singapore, China), Europe and the United States to be able to respond to growing demand for global studies. Linical provides "one stop shopping" services ranging from pharmaceutical affairs to planning, implementation plan creation, monitoring, data management, statistical analysis, and pharmacovigilance. With regards to jointly conducted global trials, Linical headquarters operates a function where personnel with in depth knowledge about various countries pharmaceutical product development work. These personnel are able to provide information necessary to establish a development environment that can enable jointly conducted global clinical trials to be conducted in Japanese. Among the new drug development projects spanning from 10 to 20 years, Linical is specialized in the processes of “Phase II” and “Phase III” that require 3 to 7 years targeting patients and are particularly important in clinical trials, and it provides “monitoring” services that are the core of the clinical trials in the contract-based business style in conjunction with “quality control” and “consulting.” It collects highly reliable data and supports the rapid and reliable development of new drugs. Furthermore, it focuses on major pharmaceutical companies with abundant drug development information and is specialized in the oncology and CNS disease realms with a strong demand for development from markets as well as the other challenging realms to respond to its customers’ needs (i.e. pharmaceutical companies).
In addition, the company offers high-quality services in the fields of schedule management, standard procedure documents for clinical trials, compliance with GCP, the reliability of data and case reports, etc.
* International joint examination
“International collaboration” refers to conducting clinical trials simultaneously in multiple countries or regions in order to develop new drugs on a global scale and aim for early launch.
*GCP (Good Clinical Practice)
“GCP” is the international rule the companies are supposed to obey when they conduct the clinical trial. It is enacted by Ministry of Health, Labor and Welfare as a ministerial ordinance so that they can conduct it properly in Japan.
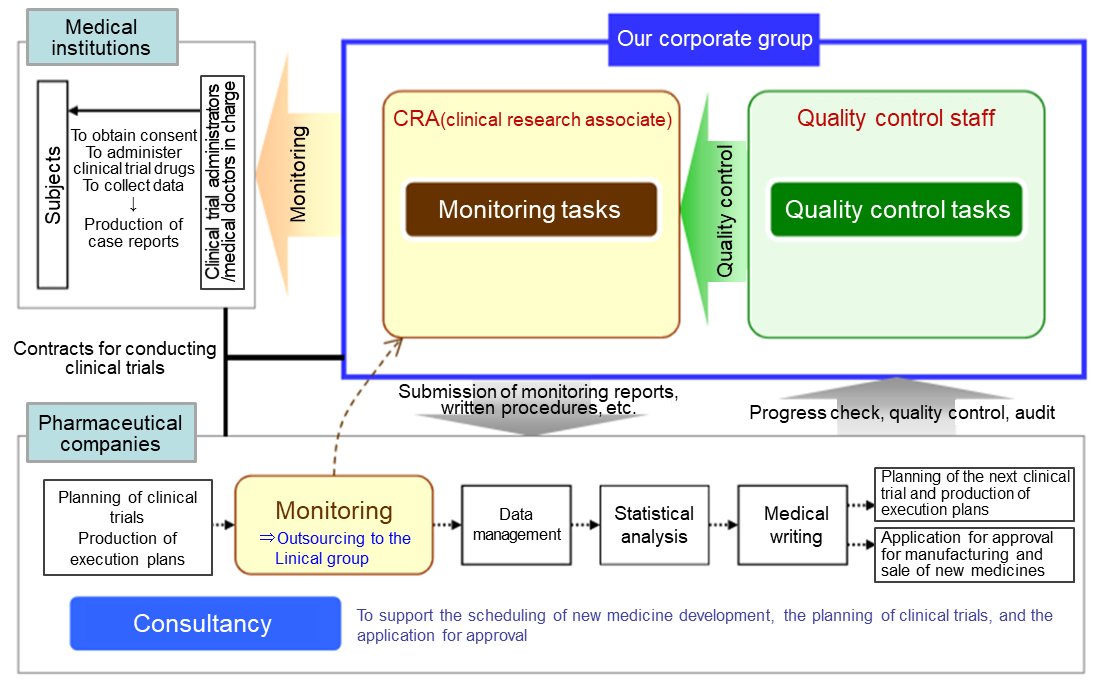
(Source Linical)
Contract Medical Affairs Business
The Clinical Trials Act is enacted, and the environment surrounding clinical research is changing drastically. Under this circumstance, to obtain information in a timely manner and be the best partner for the medical affairs department of pharmaceutical companies, Linical provides full-service support including data management and statistical analysis with a focus on monitoring and research administration works of clinical trials. It covers clinical trials that are compliant with J-GCP, ethical guidelines, the Clinical Trails Act and/or ICH-GCP, providing services for all regulations. Furthermore, it offers services in the realms of primary and CNS from the beginning of the company’s establishment. It has also strengthened the oncology realm, and more than half of the monitors are experienced in that realm. It has a policy to respond to the latest regulations and contribute to the creation of evidence in the challenging areas based on the know-how cultivated in the past development works.
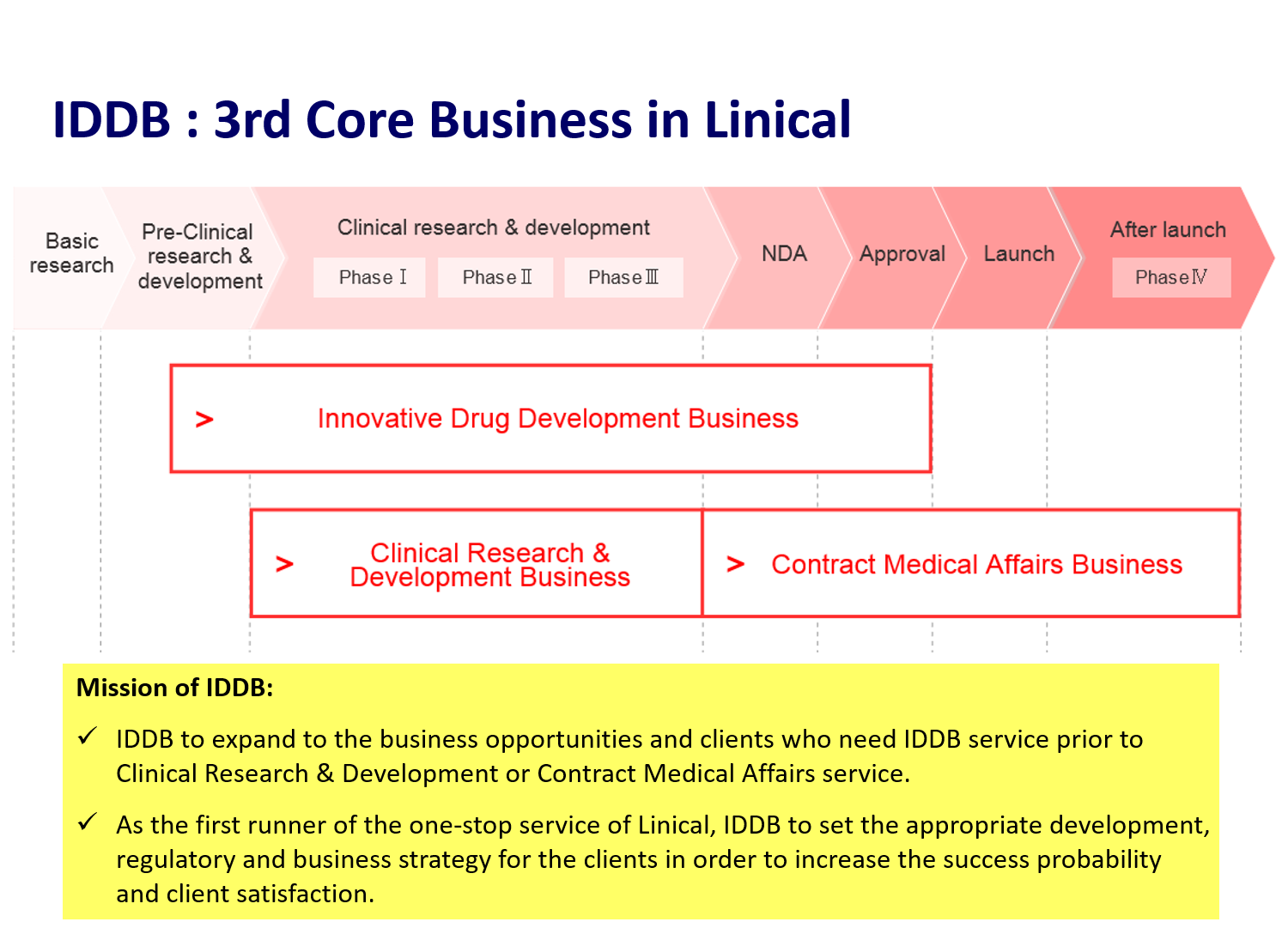
Innovative Drug Development Business
Following the existing CRO Business and Contract Medical Affairs Business, Linical is cultivating the third business called Innovative Drug Development Business. This business is mainly operated by employees who are involved with licensing, business development, clinical trials development, development pharmacy, and marketing at major domestic pharmaceutical companies and have abundant results and experiences in determining developed products, introduction and derivation negotiation, and clinical development. In the Innovative Drug Development Business, 3 types of consulting services: a) market analysis of developed products, b) support for PMDA consultations, and c) licensing support, are provided. With these experiences as a weapon, Linical is currently supporting operations of domestic and overseas pharmaceutical companies and biotechnology companies from the early stages of development. It plans to strengthen the system to provide total support globally in cooperation with its international bases.
【1-3 Five Strengths】
(1) Comprehensive "One Stop" Services on a Global Scale
Linical is Japan's only global CRO services provider with the ability to provide services in 20 different countries, mainly in the three regions of Asia, Europe, and the United States. In addition, through its partners, the company is able to provide services in about 30 different countries. Moreover, Linical boasts of highly skilled professionals with bountiful experience in a wide spectrum of comprehensive services ranging from planning, monitoring, data management, statistical analysis, medical writing, pharmaceutical affairs, pharmacology vigilance, and other various services who can respond to customers' needs, including the need for not only local but also multi-national clinical trials.
To sum up, Linical is a comprehensive "one stop" service provider operating on a global scale.
LINICAL Global Base Three Main Operating Regions of ”Japan and Asia, United States, Europe”
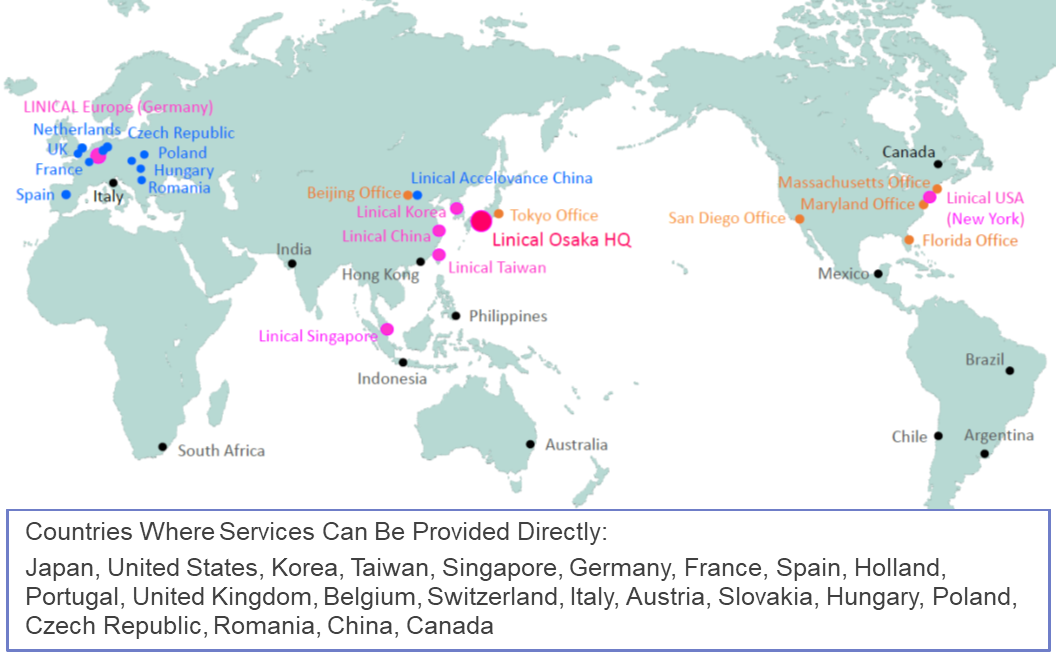
(Source Linical)
(2) Innovative Drug Discovery Support to Clinical Development and Research
Linical is a true partner that contributes to the maximization of value of drugs by offering responses required in various stages of the drug development process including new drug discovery, clinical development and post market launch manufacturing. Linical also enables clients to promote efficient new drug development, extend life cycle management, shorten the time required to market launch (TTM), and maximize sales at an early stage (TTP). In Japan, the company supports the creation of medicines in the Innovative Drug Development Business, conducts clinical development in the Contract Research Organization (CRO) business, and supports clinical tests and research after production or release in the Clinical Research Support Business.
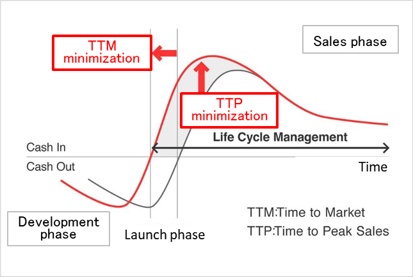
(Source Linical)
(3) Focus Upon Oncology, Central Nervous System, Immune System
Pharmaceutical product development is currently focused upon the three realms of oncology, central nervous system and immune system. Founding members of Linical boast of bountiful experience in the realm of immune system and have provided services in the highly difficult realm of immune system since the Company's founding. Thereafter, Linical has expanded its realms of expertise to include central nervous system in 2006, and oncology in 2010. Currently, Linical's business is based upon the regions of unmet medical needs in these three main business realms of oncology, central nervous system and immune system. In addition, its overseas subsidiaries are also boast of a strong track record in oncology, central nervous system, and immune system related services, which are realms where unmet medical needs are high. Furthermore, it is in the process of growing the regenerative medicine realm, which is extremely challenging, to a major pillar of future services.
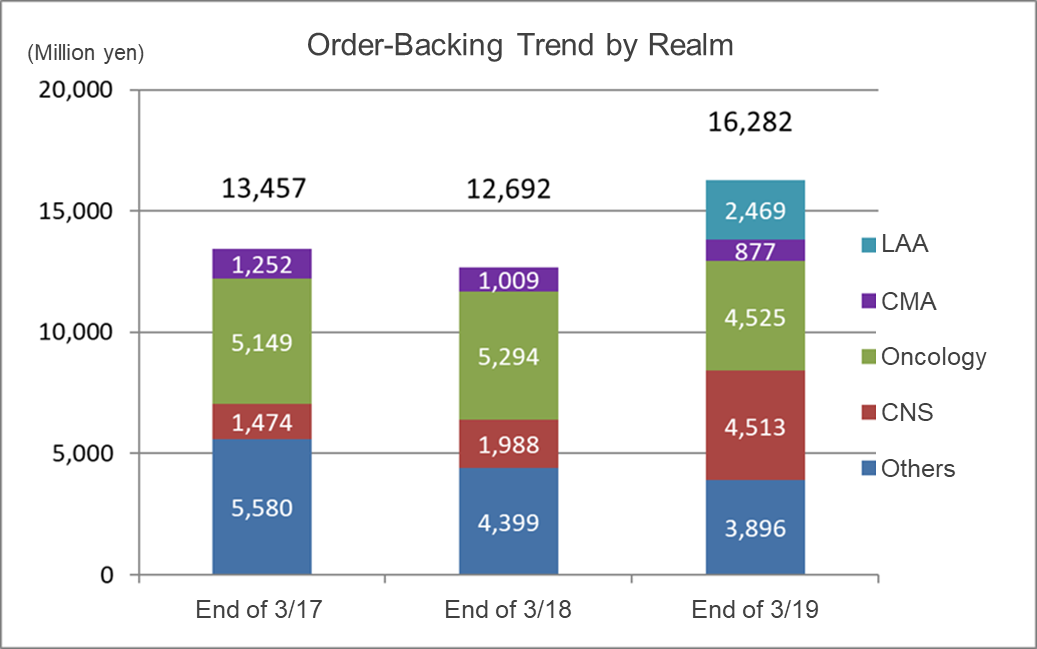
(Source: Linical)
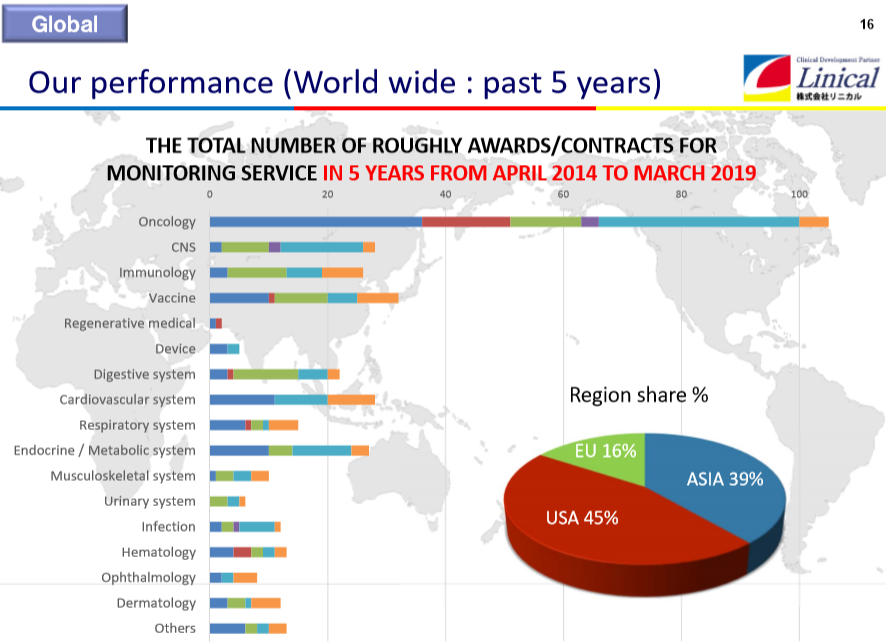
The order backlog is increasing mainly in the CNS disease realm and oncology realm. New orders are acquired while order backlog is constantly completed. Since fiscal year March 2019, the inclusion of Linical Accelovance America (LAA) in the scope of consolidation has been contributing
.
Contract test Track Record(As of November 1, 2019,)
(Source: Linical)
(4) Global Collaboration
Linical is Japan's only CRO services company that can provide clients with services on a global basis. Because of its ability to provide exceptionally high-quality services (Japan Quality), it has established its global business development center in Japan and maintains multilingual staff with the ability to communicate in Japanese, English and other languages including Korean, Taiwanese, German and others at its Osaka headquarters and Tokyo branch office. Overseas staff also understand Linical's advantage of having high quality services originating in Japan and provide these "Japan Quality" services throughout the Linical business globally.
The company offers proposals according to needs from clients, including a case in which a project manager and leaders from Japan, Taiwan, and South Korea are deployed in Japan and a case in which leaders are deployed at the footholds of respective countries for realizing a testing system in Asia, including Japan, Taiwan, and South Korea. In addition, the company has carried out many collaborative trials among enterprises in Japan, the U.S., EU, and other Asian countries, so it can give proposals on a global scale according to the development strategy of each client.
(5) High Quality Services
In order to provide high quality services that Linical is widely recognized for, the Company implements training of its staff in both aspects of quantity and quality of work. As a result, the Company has been able to maintain high passage rates of the GCP Support Certification Examinations administered by the Japan Society of Clinical Trials and Research (JSCTR) since the first examination and has been awarded with recognition awards for its high quality and passage rates, which in turn have contributed to promotion of clinical trials. In addition, Linical boasts of bountiful experience in GCP compatibility surveys and FDA inspection. In both instances, the Company has received high regard for its services from clients. Moreover, overseas subsidiaries have also received high regard for its bountiful experiences in dealings with the FDA, KFDA, ANVISA and other organizations. In addition, about 67% of trials have completed registration before the end of the predetermined registration periods. The company strongly believes that its greatest mission is to provide customers with the best service by combining high quality and speed.
2.Management Strategy
(1) Contract Research Organization (CRO) Business
Main Strategies for the CRO Business
・Establish the global structure of 1,000 members
・Promote acquisition of orders based upon comprehensive capabilities for global jointly conducted clinical trials by establishing a global structure
(Source: Linical)
(Source: Linical)
As LAA became a subsidiary of the company through acquisition, the tripolar structure that is centered in Japan and is striding across Asia, Europe, and the U.S. was fortified. In addition, a global structure composed of 1,000 staff members became feasible, and the company promoted the enhancement of marketing skills and the improvement in quality.
Global Development
【Japan】
Efforts will be focused on regenerative medicine in addition to the realms of oncology, CNS disease, and immunology as well as the expansion of the businesses in the realms of skin and eyes.
Furthermore, Linical will explore the possibilities of establishing Linical Australia and Linical South Africa to be led by Japan.
It will also expand the business in China (establishment of a subsidiary in Shanghai is completed) and dispatch employees from Japan.
【The United States】
Linical will position the base in the United States as the center of its business and nurture it. Furthermore, it will explore the possibilities of establishing Linical CANADA and expanding the businesses in Latin America.
【Europe】
It aims to improve profitability by strengthening competitiveness. In addition, it will consider the establishment of Linical Italy, reinforcement of recruitment activities for CRA in the UK, and further increasing staff and expanding bases. Also, integration of Linical Accelovance Europe and Linical Europe will be advanced.
【Korea】
Steps are being taken to expand the staff to 100 at an early stage and to establish a highly profitable earnings structure based upon two consecutive terms of profits.
【Taiwan】
In addition to acquiring new projects including subsidiaries in Singapore, it will consider expanding the businesses in Hong Kong and the Philippines.
(2) Contract Medical Affairs Business
Important strategy within the contract medical affairs business is to respond to the outsourcing needs of the growing number of corporate led clinical research related activities.
Linical differentiates itself from its competitors by focusing upon the consigned service type drug development business, mainly the provision of consigned support services for clinical research. With regards to this provision, the securing of quality levels of clinical research is a critical issue for evidence creation. Linical creates procedure manuals for structure facilitation support, monitoring and surveillance implementation. In addition, the ability to receive work for clinical research was responsible for the turn to profits of this business segment during fiscal year March 2013, and new orders for clinical research and development in fiscal year March 2014 allowed sales and profits to rise from the previous term.
Linical plans to continue to carry out active recruitment activities to respond to vigorous inquiries from potential clients, taking advantage of the implementation of the Clinical Trials Act.
Earnings Trend of the pharmaceutical business
| FY 3/ 11 | FY 3/ 12 | FY 3/ 13 | FY 3/ 14 | FY 3/ 15 | FY 3/ 16 | FY 3/ 17 | FY 3/ 18 | FY 3/ 19 |
Sales | 97,827 | 138,400 | 169,226 | 288,205 | 364,918 | 553,399 | 806,764 | 908,810 | 954,438 |
Operating Income | -15,834 | -21,016 | 19,504 | 68,010 | 111,006 | 208,284 | 293,028 | 288,121 | 313,911 |
* Unit: thousand yen
(Note) From the first quarter of FY 3/16, the name of the previous segment was changed from “CSO business” to “Contract Medical Affairs business ”.
Acceptance of the clinical research (as of November 1, 2019)
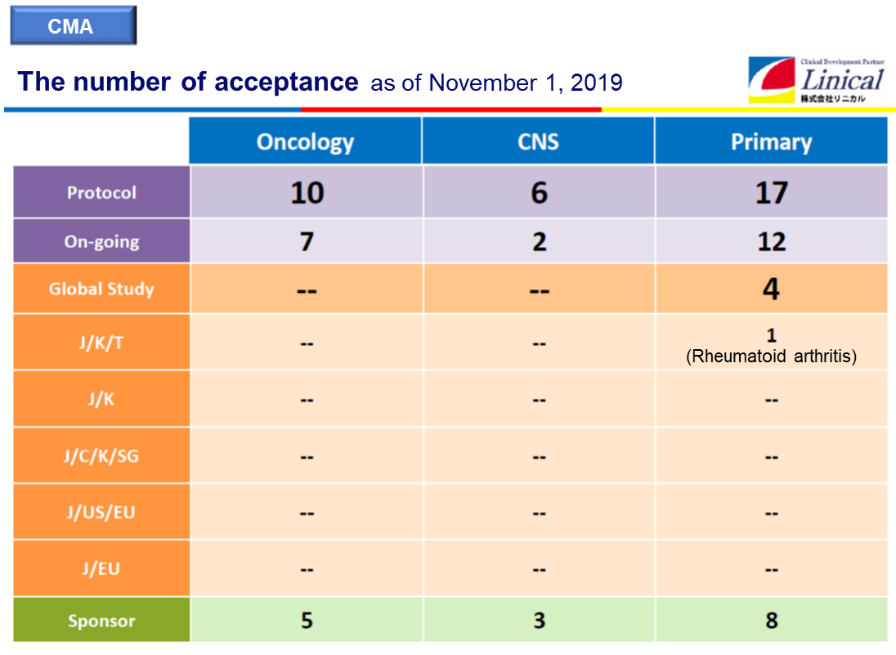
(Source: Linical)
(3) Innovative Drug Development Business (New Business Development)
Important strategies within the innovative drug development business include providing a wide range of services that respond to development plan establishment and drug authority correspondence, and leveraging new drug development funds.
Administrative authorities in Japan have needs of putting innovative medicines and medical equipment from Japan into practice earlier than any other countries in the world. In addition, administrative authorities in Korea and Taiwan have needs of creating new medicine that would enhance their international competitiveness. Furthermore, pharmaceutical companies and biotech venture companies that are Linical’s customers are interested in entering the Japanese pharmaceutical market to distribute and sell their products, but they are faced with challenges such as unfamiliarity with the Japanese market and pharmaceutical affairs, insufficient development capabilities, and lack of strategic partners/licensees. In response to these recent needs, Linical is strengthening its Innovative Drug Development Business to make it a third business. Linical can provide not only clinically developed products but also support services by professionals who have a long experience of providing support services at the earlier phase and, “research,” “development” and “licensing” at major pharmaceutical companies, including the partnership with both domestic and foreign biotech venture companies. Linical also finances new drug development funds. The increase of investment projects from new drug investment funds leads to the expansion of its subsequent CRO business, and its judging capability cultivated in the experiences so far will be made used of effectively. Furthermore, experiences including development plan establishment and drug correspondence are expected to accumulate.
(Source: Linical)
Innovative Drug Development Business -3 types of consulting-
Market analysis | ・Patient forecast ・Current market value and forecast ・Current treatment algorithm and guidelines ・Approved products and major pipelines ・Target product profile ・Price and Sales forecast of the client’s product |
Development & Regulatory Strategy/PMDA Consultation/ MW | ・Proposal of the development and regulatory strategy ・Application of the meeting to PMDA and preparation of the materials ・Presence at the meeting with PMDA w/wo the client ・Writing the IB, Protocol and ICF ・In-Country Clinical Caretaker ・Response to queries from MHLW/PMDA ・Orphan designation registration ・Master File registration |
Strategic Partnering/ Licensing | ・Search and analysis of potential partners ・Approach to potential partners and introduction of the client’s product ・Arranging the meeting and mediating communication between the client and potential partners ・Attendance of partnering conferences such as Bio Intl. Convention w/wo the client ・Support of due diligence and term discussion |
Contract in Innovative Drug Development Business – (from October 2016 to October 2019)-
Product/Skill | disease | The nationality of contract company | The development level in the most leading country | Service contents | ||
Market analysis | Pharmaceutical affair・MW | strategic cooperation /license | ||||
Nucleic acids | Respiratory diseases | Country A* | Phase I | ● |
| ● |
Mesenchymal stem cells | Immune diseases | Country B | Phase II | ● | ● | ● |
Monoclonal antibodies | Infection | Country C | Non-clinical | ● |
| ● |
Monoclonal antibodies | Malignant tumors | Country A* | Non-clinical | ● | ● |
|
Imaging tracer | Neurodegenerative diseases | Country C* | Phase I |
| ● |
|
Low-molecular compounds | Eye diseases | Country A | Phase I |
| ● |
|
Digital apps for treatment | Psychosomatic medicine | Country A | Phase Ⅲ |
| ● |
|
Immunotherapy | Allergy diseases | Country D | Phase I/II |
| ● |
|
Monoclonal antibodies | Malignant tumors | Country E | Phase Ⅲ | ● | ● | ● |
Topical medicaments | Nerve pain | Country A | Non-clinical | ● | ● |
|
Topical medicaments | Skin diseases | Country A | Application being prepared |
| ● |
|
Nucleic acids | Inflammation/infection/ ophthalmology | Country A* | Non-clinical |
| ● |
|
Low-molecular compounds | Nerve pain | Country A | Phase I |
| ● |
|
Genetically engineered biological products | Inflammatory neurological diseases | Country A | Application being prepared |
| ● |
|
Low-molecular compounds | Gastrointestinal diseases | Country F | Phase III/Ⅲ |
| ● |
|
Low-molecular compounds | Neurodegenerative diseases | Country G | Phase I/II |
| ● | ● |
●:Service being offered ●:Service provision ended
(Source: Linical)
3.The First Half of Fiscal Year March 2020 Earnings Results
(1) Consolidated results
| 1H of FY 3/ 19 | Ratio to sales | 1H of FY 3/ 20 | Ratio to sales | YoY |
Sales | 5,612 | 100.0% | 5,389 | 100.0% | -4.0% |
Gross profit | 2,047 | 36.5% | 1,835 | 34.0% | -10.4% |
SG&A | 1,401 | 25.0% | 1,264 | 23.5% | -9.8% |
Operating Income | 646 | 11.5% | 571 | 10.6% | -11.7% |
Ordinary Income | 748 | 13.3% | 484 | 9.0% | -35.2% |
Parent Net Income | 386 | 6.9% | 230 | 4.3% | -40.3% |
*Unit: million yen
* The figures include figures calculated by Investment Bridge Co., Ltd., and may differ from actual figures (Abbreviated hereafter)
Sales and ordinary income dropped 4.0% and 35.2%, respectively, year on year.
Sales were 5,389 million yen, down 4.0% year on year, while ordinary income was 484 million yen, down 35.2% year on year.
The scales of the CRO and CSO markets, where the company operates business, are growing gently, as the development and sale of pharmaceuticals are increasingly outsourced and international collaborative tests are increasing. In addition, pharmaceutical companies are estimated to outsource the development and sale of pharmaceuticals more frequently, in order to create innovative medicines and improve their productivity and efficiency. Under these circumstances, the company offers its services in Europe, the U.S., and Asia, mainly Japan, to meet the needs from pharmaceutical companies for the development of pharmaceuticals on a global scale.
The sales in Japan, Europe, South Korea, and Taiwan were in line with the initial estimates as a whole. In the U.S., the efforts for strengthening management, enhancing the marketing capability, and restructuring the organization, which were initiated in the previous term, started paying off, cementing a foothold for recovery from the second half. In China, a subsidiary directly managed by the company started business operation. Despite such a favorable situation, the sales of overseas subsidiaries in Japanese yen decreased due to the yen appreciation, and several large-scale projects ended in Japan from the previous term to this term and new projects are still to be started.
Operating income was in line with the initial estimate as a whole like sales, but decreased 11.7% year on year to 571 million yen, because the company paid lawyers’ remunerations for negotiating about the closing price of a subsidiary in the U.S. with the seller. Gross profit rate dropped 2.5 points year on year to 34.0%, SGA decreased 9.8% year on year, and the ratio of SGA to sales declined 1.5 points to 23.5%. As a result, operating income rate dropped 0.9 points to 10.6%. Ordinary income dropped 35.2% year on year to 484 million yen, as an exchange gain of 116 million yen was posted in foreign currency deposits due to the yen depreciation in the same period of the previous year while an exchange loss of 63 million yen was posted in foreign currency deposits in this term due to the yen appreciation. In addition, profit attributable to owners of parent decreased 40.3% year on year to 230 million yen, as the company paid 55 million yen as lawyers’ remunerations for arbitration in the U.S. and posted it as an extraordinary loss.
*At the time of the business combination with LAA implemented on April 16, 2018, the company conducted provisional accounting for the first half of the previous term. At the end of the previous term, the allocation of acquisition costs to assets and liabilities was finished, so the results for the first half of the previous term were revised retroactively. The results for the first half of this term were compared with the revised results for the first half of the previous term.
Sales and profit by segment
| 1H of FY 3/ 19 | Ratio to sales | 1H of FY 3/ 20 | Ratio to sales | YoY |
CRO business | 5,116 | 91.2% | 4,948 | 91.8% | -3.3% |
CMA business | 495 | 8.8% | 441 | 8.2% | -10.9% |
Consolidated sales | 5,612 | 100.0% | 5,389 | 100.0% | -4.0% |
CRO business | 1,329 | 88.0% | 1,179 | 87.6% | -11.3% |
CMA business | 182 | 12.0% | 166 | 12.4% | -8.3% |
Adjustment amount | -865 | - | -775 | - | - |
Consolidated operating income | 646 | - | 571 | - | -11.7% |
*Unit: million yen
The sales of the CRO business was in line with the initial estimate as a whole, but declined 3.3% year on year, as the yen appreciation decreased the sales of overseas subsidiaries in Japanese yen and several large-scale projects ended in Japan from the previous term to the first half of this term and new projects are still to be started. As sales decreased year on year, operating income declined 11.3% year on year. The profit rate of this segment dropped 2.2 points year on year to 23.8%.
The sales of the Contract Medical Affairs Business decreased 10.9% year on year, as the existing projects ended, new products were still to be started, and relatively large sales were posted in the previous term, in which large-scale projects began. Operating income declined 8.3% year on year, as the rate of utilization of personnel declined due to the drop in sales. The profit rate of the segment rose 1.1 points year on year to 37.8%.
*At the time of the business combination with LAA implemented on April 16, 2018, the company conducted provisional accounting for the first half of the previous term. At the end of the previous term, the allocation of acquisition costs to assets and liabilities was finished, so the results for the first half of the previous term were revised retroactively. The results for the first half of this term were compared with the revised results for the first half of the previous term.
(2) Non-consolidated performance trends in each country
| 1H of FY 3/19 | 1H of FY 3/20 | ||||
sales | Ordinary profit | sales | YoY | Ordinary profit | YoY | |
Japan | 3,814 | 1,062 | 3,694 | -3.1% | 573 | -46.1% |
Consolidated performance in the U.S. (LAA + LAE + LAC) | 996 | -383 | 982 | -1.4% | -267 | - |
Consolidated performance in Europe (LEU only) | 945 | 72 | 1,056 | +11.7 | 123 | +71.5% |
South Korea | 204 | 17 | 239 | +17.1% | 62 | +249.3% |
Taiwan | 126 | 29 | 121 | -4.5% | 25 | -12.6% |
China (Linical China only) | - | - | 9 | - | -2 | - |
Adjustment | -476 | -50 | -714 | - | -30 | - |
Total | 5,612 | 748 | 5,389 | -4.0% | 484 | -35.2% |
*Unit: million yen, %
The results in the U.S., Europe, South Korea, and Taiwan were in line with the company’s estimates as a whole, but affected by the yen appreciation.
(3) Change in order balance
| End of FY 3/19 (A) | End of 1H of FY 3/20 | As of Nov. 14, 2019 (B) | Difference from the end of the previous term (B-A)/(A) |
Eisai | 3,350 | 4,379 | 4,259 | +27.1% |
Chugai Pharmaceutical | 3,579 | 2,867 | 2,717 | -24.1% |
Ono Pharmaceutical | 2,476 | 1,781 | 1,661 | -32.9% |
Other | 6,876 | 9,386 | 10,655 | +55.0% |
Total backlog of orders | 16,282 | 18,414 | 19,294 | +18.5% |
*Unit: million yen
Order-Backing Trend by country
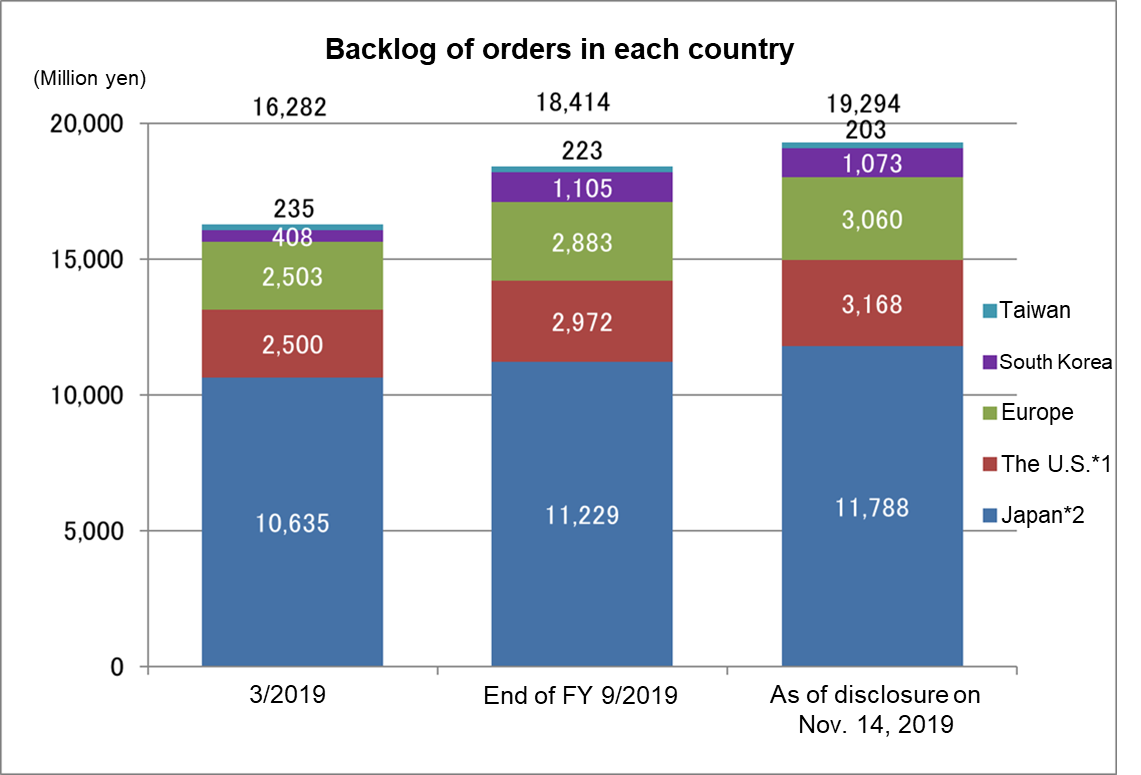
* 1 The backlogs of orders of the European and Chinese subsidiaries of Linical Accelovance America are included in the backlog of orders in the U.S.
* 2 The backlog of orders of Linical China Co., Ltd. is included in the backlog of orders in Japan.
In the CRO Business, the total amount of a contract is determined based on the number of cases and the difficulty of trials based on the target disease during the trial period of about one to three years. A contract is signed with a client for this trial period, and sales are booked on a monthly basis in line with consignment contracts. Also, in the CMA Business, a contract is signed with a client for almost the same period, and sales are booked on a monthly basis in line with consignment contracts. Consequently, the total order backlog reflects the residual value of consignment contracts already concluded. Therefore, the order backlog reflects sales that will be booked during the course of the next one to five years and are used as an assumption for estimates of future earnings.
Order backlogs as of November 14, 2019, were 18.5% higher than those at the end of the previous fiscal year (March 2019). This is because although existing contracted contracts were steadily completed and the order balance was recorded as sales, there were new contracts for contracted projects that exceeded them.
In detail, the company received a new order from an overseas bio-venture firm for large-scale clinical trials for which the company will serve as a clinical caretaker in Japan, and new orders for clinical trials in Japan from leading overseas pharmaceutical companies. In addition, some pharmaceutical companies and bio venture firms in Japan, the U.S., etc. have decided to place orders, and when taking into account the projects for which contracts are being drafted, the backlog of orders is virtually over 20 billion yen. As outsourcing and international collaborative trials are increasing, the recent environment for order receipt has been favorable. Existing and new clients have contacted the company for placing orders thanks to the marketing activities. Accordingly, the company plans to fortify the system for undertaking tasks by increasing CRA staff and so on.
(4) Financial Conditions and Cash Flow(CF)
Financial Conditions
| March 2019 | September 2019 |
| March 2019 | September 2019 |
Cash | 5,055 | 4,988 | ST Interest-Bearing Liabilities | 1,619 | 1,529 |
Receivables | 1,602 | 1,683 | Payables | 963 | 956 |
Advance payment | 663 | 720 | Taxes Payable | 488 | 294 |
Current Assets | 7,723 | 7,845 | LT Interest-Bearing Liabilities | 3,105 | 3,353 |
Tangible Assets | 134 | 657 | Liabilities | 8,008 | 8,541 |
Intangible Assets | 4,461 | 4,173 | Net Assets | 5,250 | 5,070 |
Investments and Others | 939 | 935 | Total Liabilities and Net Assets | 13,259 | 13,612 |
Noncurrent Assets | 5,535 | 5,766 | Total Interest-Bearing Liabilities | 4,725 | 4,883 |
* Unit: million yen
* Interest-bearing liabilities=Borrowings + Lease Obligations
The total assets as of the end of September 2019 were 13,612 million yen, up 352 million yen from the end of the previous term. On the side of assets, receivable, advance payment, tangible assets, etc. are major factors in increase, while on the side of liabilities and net assets, advances received, deposits, and lease obligations, etc. are major factors in increase. The goodwill as of the end of September 2019 was 3,961 million yen, down 273 million yen from the end of the previous term. The capital-to-asset ratio as of the end of September 2019 was 37.2%, down 2.4 points from the end of the previous term.
Cash Flow
| 1H of FY 3/ 19 | 1H of FY 3/ 20 | YoY | |
Operating cash flow(A) | -421 | 581 | 1,003 | - |
Investing cash flow (B) | -2,518 | -99 | 2,418 | - |
Free cash flow(A+B) | -2,940 | 481 | 3,422 | - |
Financing cash flow | 3,523 | -580 | -4,104 | - |
Cash and Equivalents at the end of term | 5,718 | 4,988 | -730 | -12.8% |
* Unit: million yen
As for cash flows, operating CF turned positive, due to the increase in reserve for bonuses, the shrinkage of the decrease rate of other accounts payable, the increase in accrued expenses, the rise in advances received, etc. from the same period of the previous year. In addition, the deficit of investing CF shrank as there was no longer the purchase of shares of subsidiaries resulting in change in scope of consolidation, and free CF turned positive. On the other hand, financing CF turned negative due to the reduction of short-term debts, etc.
(5) Business combination, etc.
(Regarding the important revision to the initial allocation of acquisition costs for compared information)
At the time of the business combination with LAA implemented on April 16, 2018, the company conducted provisional accounting for the first half of fiscal year March 2019. At the end of fiscal year March 2019, the allocation of acquisition costs to assets and liabilities was finished. The comparison information in the financial statements for the first half of this term reflects the important revision to the initial allocation of acquisition costs. Mainly, 16,082 thousand yen was allocated to assets related to the backlog of orders, which are intangible assets, and 92,740 thousand yen was allocated to assets related to clients, and other accounts payable of 64,164 thousand yen were recognized. As a result, the goodwill amount increased 62,374 thousand yen from 3,356,005 thousand yen, which was provisionally estimated, to 3,418,379 thousand yen. Consequently, in the profit-and-loss statement for the first half of the previous term, operating income, ordinary income, and net income before taxes and other adjustments decreased 5,313 thousand yen each, and profit attributable to owners of parent declined 3,058 thousand yen. The price adjustment after the acquisition of shares has not been completed, so the goodwill amount may change according to the results of the price adjustment.
4.Fiscal Year ending March 2020 Earnings Forecasts
(1) Consolidated results
| FY 3/19 Act. | Ratio to sales | FY 3/20 Est. | Ratio to sales | YoY |
Sales | 11,313 | 100.0% | 11,700 | 100.0% | +3.4% |
Operating Income | 1,212 | 10.7% | 1,560 | 13.3% | +28.6% |
Ordinary Income | 1,253 | 11.1% | 1,538 | 13.1% | +22.8% |
Parent Net Income | 568 | 5.0% | 971 | 8.3% | +70.9% |
* Unit: million yen
Linical’s Earnings Estimates Call for Sales, Ordinary Income to Rise 3.4% and 22.8% Year-On-Year
After the first half of this term, the corporate plan for fiscal year March 2020 has not been revised, with the target sales being 11.7 billion yen, up 3.4% year on year, and ordinary income being 1,538 million yen, up 22.8% year on year.
With regard to sales, Linical will endeavor to acquire repeat orders from existing clients who have high regard for Linical’s businesses and to strengthen marketing activities to secure orders from new clients within the CRO Business. In particular, Linical intends to expand sales by acquiring new projects including global joint clinical trials in the oncology and CNS disease realms, where there is strong demand for new drug development and the Linical Group has high expertise. In the Contract Medical Affairs Business, Linical will expand its customer base by vigorously implementing marketing activities with a focus on company-driven clinical research after the launch of new drugs and strive to obtain new projects in the realms where it has expertise utilizing the know-how gained in the CRO Business.
With regard to profit, in the CRO Business, in addition to the amortization of goodwill associated with the M&A of European subsidiaries, the amortization of goodwill associated with the acquisition of LAA in April 2018 will be added. However, Linical will take measures to realize a highly profitable earning structure with increasing number of global jointly conducted clinical trials by strengthening management base of the overseas subsidiaries and expanding the businesses in North America. In the Contract Medical Affairs Business, an increase in sales due to acquisition of new projects is expected to contribute to an increase in profit.
Operating income is expected to grow by 28.6% year-on-year to 1,560 million yen. Despite the upfront investments including increases in staffing and consolidation of subsidiaries in Europe, taking into account the effects of the structural reforms implemented in the previous fiscal year, operating income margin is projected to rise 2.6 points to 13.3%. The company does not anticipate any significant amounts of income or losses to be booked at the non-operating and extraordinary income levels. As for dividends, the plan to pay 14 yen/share, up 2 yen/share from the previous term, is unchanged. Namely, the ordinary dividend will be 13 yen/share, up 1 yen/share from the previous term, and the company will also pay a commemorative dividend of 1 yen/share as the consolidated sales for fiscal year March 2019 exceeded 10 billion yen.
(2) Progress of the performance in the first half (the cumulative second quarter) toward the full-year forecast
| 1H of FY 3/20 | Company Forecast of FY 3/20 | Progress rate |
Sales | 5,389 | 11,700 | 46% |
Operating Income | 571 | 1,560 | 37% |
Ordinary Income | 484 | 1,538 | 32% |
Parent Net Income | 230 | 971 | 24% |
*Unit: million yen
The sales and profits in the consolidated cumulative second quarter of fiscal year March 2020 are all less than 50% of the annual estimates for this term. However, the performance of the company tends to be better in the second half every year, because the orders received in the first half will contribute and the utilization rate of CRA recruited in the first half will increase in the second half.
(3) Balance of goodwill and the remaining amortization period (as of the end of fiscal year March 2019)
| Amount | Period | the amount of amortization per year |
South Korea | Amortization Finished | ||
Europe ※1 | 921 | 14 years | 69 |
The U.S. ※2 | 3,313 | 15 years | 221 |
*Unit: million yen
The amortization of goodwill of the subsidiary in South Korea will end, and profitability is expected to improve significantly this term.
※1 The balance of intangible assets recognized through Purchase Price Allocation other than goodwill at the end of FY 3/19 is 89 million yen. The remaining period for the amortization is 12 years (8 million yen per year.)
※2 The balance of intangible assets recognized through Purchase Price Allocation other than goodwill at the end of FY 3/19 was 96 million yen. For 11 million yen out of them, the remaining amortization period is 2 years (annual amortization amount: 5 million yen). For 85 million yen out of them, the remaining amortization period is 8 years (annual amortization amount: 10 million yen).
※3 The price adjustment after the acquisition of shares has not been finished, and the goodwill as of the end of fiscal year March 2019 is a provisionally calculated amount.
5.Conclusions
The company receives an increasing number of orders. This term, the company received a new order from an overseas bio-venture firm for large-scale clinical trials for which the company will serve as a clinical caretaker in Japan, and orders for clinical trials in Japan from leading overseas pharmaceutical companies. In addition, some pharmaceutical companies and bio venture firms in Japan, the U.S., etc. have decided to place orders, and when taking into account the projects for which contracts are being drafted, the backlog of orders is virtually over 20 billion yen. This can be said to be the outcome of the establishment of the global system for undertaking projects based in the U.S., Europe, and Asia, including Japan and China. The large-scale order received this term will contribute to the performance in the next term or later rather than the second half of this term. As only the Linical Group undertakes large-scale international collaborative trials, the average price per transaction will increase significantly, and this is expected to become a growth driver for the company. The trend of order receipt in the second half of this term, which will become a leading indicator for the future performance, is noteworthy. Especially, we would like to pay attention to whether the company can continuously receive orders for large-scale international collaborative trials with expectation.
Since the start of the business, the company has conducted the CRO business while specializing in cancer, the central nervous system, and the immune system. The company will continue the business while focusing on these three fields, but plans to enter the fields of regenerative medicine, dermatology, and ophthalmology, where the number of clinical trials is expected to increase considerably. Their growth strategies in the promising fields of regenerative medicine, dermatology, and ophthalmology are noteworthy.
<Reference: Regarding Corporate Governance>

◎Corporate Governance Report
The company submitted its latest corporate governance report on July 2, 2019 after applying the corporate governance code
<Basic Policy>
The company will contribute to the development of pharmaceutical products as a partner of leading pharmaceutical companies in Japan with its technology for developing pharmaceutical products and live up to the expectations of the entire society from the pharmaceutical field. In addition, in order to improve corporate value, it is necessary to establish a system for making decisions swiftly while securing soundness and transparency.Therefore, the company plans to reinforce its internal control, including thoroughgoing compliance with laws, which is the most important issue to be solved. Based on this idea, we are strengthening the internal control, including thorough compliance, which is the most important issue.
<Regarding the implementation of the principles of the corporate governance code>Major principles and reasons
Principles | Reasons for not implementing the principles |
Principle 4-7 Roles and Responsibilities of Independent External Directors | To secure effective management supervision from a standpoint independent from management, etc., and enhance corporate value over the medium to long term by obtaining advice based on the extensive experiences and knowledge, we appoint two external directors who are familiar with the pharmaceutical industry and have deep knowledge and experiences. We will build a system in which they can fulfill their roles and responsibilities. |
【Supplementary Principle 4-8-2 Utilization of independent external directors】 | Our company will develop systems for communication and coordination between independent external directors and the management and for cooperation with auditors or the board of auditors. |
【Principle 4-9 Criteria for judging the independence of independent outside directors and their qualities】 | Our company will carefully discuss the disclosure of criteria for objectively judging independence, etc. that have no risk of causing a conflict of interest with general shareholders. |
【Supplementary Principle 4-10-1 Utilization of arbitrary systems】 | In our company, the number of independent outside directors is 2, systems for communication and coordination among directors and the management and for cooperation with auditors have been established, and sufficient discussions about nomination, remunerations, etc. are made by the board of directors. The establishment of arbitrary committees for nomination and remunerations will be discussed when necessary. |
<Disclosure Based on the Principles of the Corporate Governance Code (Excerpts)>
Principles | Disclosure contents |
| <Principle 1-4 Strategically held shares>
| In order to avoid the risk of share price fluctuations and also improve capital efficiency, the company will not hold any listed shares, unless it is necessary to hold shares for cooperation and alliance. |
Supplementary principle 4-11-3 Prerequisites for ensuring effectiveness of the Board of Directors and Board of Auditors
| As a result of analyzing and evaluating the effectiveness of the Board of Directors by the Board of Directors, we have concluded that it is operating effectively as follows. During the previous fiscal year, the Board of Directors met 13 times in total, including regular and extraordinary meetings, and various management issues were discussed including management strategy and investment, and business execution. 1) In accordance with the rules of the Board of Directors, every important matter is selected as agenda, and the Board of Directors meets every month for deliberation in a timely and appropriate manner. 2) Prior to deliberation at the Board of Directors meetings, problems and issues, risks, and countermeasures are clarified at management meetings such as the Management Committee to increase the effectiveness of discussions. 3) The information materials for the Board of Directors meetings are distributed in advance for smooth and active discussions and for sufficient deliberation at the Board of Directors. 4) In principle, all directors, all external auditors (independent auditors), and observers including advisory lawyers, all executive officers, and general manager of the Corporate Planning Department attend the Board of Directors meeting every month to actively express their opinions to that the objectivity of the discussion is enhanced. 5) We receive reports on a regular basis concerning the management status through various conference bodies and implement appropriate risk management and business execution monitoring. 6) In order to further enhance the management supervisory function of the Board of Directors, we consider it important to have external directors with extensive management experiences. Therefore, from the previous fiscal year, we increased the number of external directors to 2, and a total of 5 independent officers, including 3 external auditors, participate in the monthly Board of the Directors meetings in principle, and actively express their opinions, thereby increasing the objectivity of the discussion. |
Supplementary principle 4-14-2 Training of Directors and Auditors
| We not only encourage the Directors and Auditors to proactively learn but provide workshops on the themes (e.g. compliance, trends at general meetings of shareholders) that are considered necessary for the performance of their duties. In addition, we offer opportunities for office visits, conference tours, and interviews for them to understand the status of business execution. Information on workshops held by third parties that are useful for the performance of their duties is also shared with the Directors and Auditors. |
<Principle 5-1 Policy for constructive dialogue with shareholders> | The company has continuous, constructive, transparent, fair dialogue regarding business performance, managerial strategies, capital policies, risks, corporate governance systems, etc. with the following method, in order to foster trusting relationships with the aim of achieving the sustainable growth of corporate value, which is a shared goal of the company and shareholders (including potential institutional and individual investors).
Dialogue with shareholders is led by the Managing Director CFO. Considering the purpose and effect of the interview, and the attributes of shareholders, the dialogue method is examined thoroughly by the senior management such as CEO and the Managing Director CFO. As for IR, mainly the financial affairs department and the management planning division gather necessary information from relevant sections of the company, prepare reference material and give explanations in an understandable manner, to enrich the dialogue with shareholders.The company has opportunities to dialogue with shareholders through the annual general meetings of shareholders, results briefing sessions (twice a year), briefing sessions for individual investors (twice a year), meetings with institutional investors and analysts inside and outside Japan at the time of disclosure of quarterly results, the disclosure of IR information via websites, the response to inquiries from individual investors by telephone, email, or the like. Then, the company reflects questions, requests, information on participants in briefing sessions, questionnaire results, etc. in IR activities.Shareholders’ interests and concerns grasped through the dialogue with them are reported to the senior managing director CFO and the information is utilized for analyzing business administration, discussing how to disclose information, etc.Concerning IR activities and the dialogue with shareholders, the company manages insider information appropriately in accordance with in-company rules. The quiet period, in which the company refrains from having dialogue about financial results, is from the day after the closing date of each quarter to the date of brief reporting. |
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However, we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration. Copyright(C) 2019 Investment Bridge Co., Ltd. All Rights Reserved. |
To view back numbers of Bridge Reports on Linical Co., Ltd. (2183) and other companies and to see IR related seminars of Bridge Salon, please go to our website at the following url: www.bridge-salon.jp/


